Page 52 - Application Notebook - Solution for Food Safety
P. 52
LAAN-A-LM-E109
Application Liquid Chromatography Mass Spectrometry
News Multi-Residue Analysis of 18 Regulated Mycotoxins
by LC/MS/MS
No.C138 D. Baker , C. Titman , J. Horner , N. Loftus :
2
1
1
1
2
1
Shimadzu UK, Scientific Analysis Laboratories
Mycotoxins are one of the most important contaminants Due to the wide range of physicaland chemical
in food and feed due to their widespread distribution in properties of mycotoxins, different LC/MS/MS methods
the environment and toxic effects on humans and are typically developed for small groups of compounds
1)
animals. Structurally, mycotoxins are a very diverse with similar properties.
group with a wide range of physicochemical properties
2)
and low molecular weights. They are produced by fungi In this application paper a single LC/MS/MS method has
(mould) frequently found on agricultural produce, and been developed for the determination of 18 mycotoxins
3)
are often not visible to the naked eye. Some of the in food safety. Limits of quantification were at or below
most commonly contaminated food stuffs include the maximum levels set in the EC/1886/2006 document.
4)
wheat, oats, rye, corn, barley, rice, nuts and milk. The scope of the method included Aflatoxins (B1, B2,
G1, G2), Fumonisins (B1, B2, B3), Ochratoxin A (OTA)
Due to the risks posed by mycotoxins in food they are and Trichothecenes (3-acetyldeoxynivalenol (3AcDON),
regulated globally, including, the EU, US, China, 15-acetyldeoxynivalenol (15AcDON), Deoxynivalenol
5)
Singapore and Brazil. In the EU, reporting limits are (DON), Diaceteoxyscripanol (DAS), Fusarenon-X (FUS X),
harmonised in Regulation (EC) No 1886/2006 (amended HT-2, Neosolaninol (NEO), Nivalenol (NIV), T2,
by (EC) No 1126/2007) and sampling and analysis in Zearalenone (ZON)) with an analysis cycle time of
Regulation (EC) No 401/2006. 12.5 minutes.
LC/MS/MS is the technique most commonly employed Table 1 Analytical Conditions
for mycotoxin quantitation in order to achieve the UHPLC : Nexera LC System
necessary low reporting limits in complex food and feed Mobile Phase : A; Water with additives
matrices. B; Methanol with additives
Column : Reversed phase column (100 mm L.× 2.1 mm I.D.)
Q Experimental Column Temperature : 40 ˚C
Flowrate : 0.4 mL/minute
Solvent extracts were provided by Scientific Analysis Gradient B. Conc 15 % (0 min) ˠ 25 % (1 min)
Laboratories (SAL, UK) following validated extraction ˠ 40 % (2 min) ˠ 41 % (4.5 min)
protocols. Samples were analysed using the Nexera ˠ 100 % (7.5 - 10.0 min) ˠ 15 % (10.10 min)
Stop (12.5 min)
UHPLC and the LCMS-8060 triple quadrupole detector LC-MS/MS : ˠ LCMS-8060
13
(Table 1) . Calibration was performed using C internal Dwell Time : 10 to 40 msec.
standards spiked during sample extraction. All MRM Pause Time : 1 msec.
transitions and associated internal standards for each Ionisation Mode : ESI +/-
compound are listed in Table 2. All solvents used during Polarity Switching : 5 msec.
analysis were LCMS quality from Sigma-Aldrich.
(×1,000,000)
1.2
NEO ZON
1.1 OTA
1.0
DON FUS-X DAS
0.9 T2
0.8
0.7
0.6 AFB2 AFB1 FB2
15-AcDON
0.5
0.4 AFG1 FB3
0.3 3-AcDON AFG2 FB1
0.2 HT-2
0.1
NIV
0.0
0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0 4.5 5.0 5.5 6.0 6.5 7.0 7.5 min
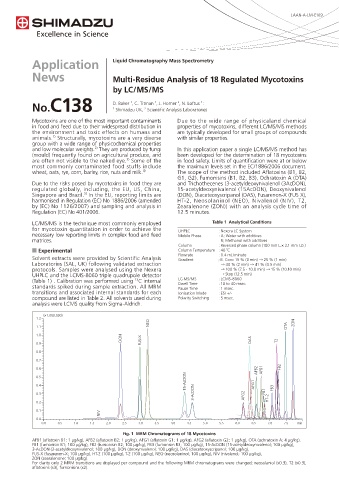
Fig. 1 MRM Chromatograms of 18 Mycotoxins
AFB1 (aflatoxin B1; 1 μg/kg), AFB2 (aflatoxin B2; 1 μg/kg), AFG1 (aflatoxin G1; 1 μg/kg), AFG2 (aflatoxin G2; 1 μg/kg), OTA (ochratoxin A; 4 μg/kg),
FB1 (fumonisin B1; 100 μg/kg), FB2 (fumonisin B2; 100 μg/kg), FB3 (fumonisin B3; 100 μg/kg), 15-AcDON (15-acetyldeoxynivalenol; 100 μg/kg),
3-AcDON (3-acetyldeoxynivalenol; 100 μg/kg), DON (deoxynivalenol; 100 μg/kg), DAS (diaceteoxyscripanol; 100 μg/kg),
FUS-X (fusarenon-X; 100 μg/kg), HT-2 (100 μg/kg), T-2 (100 μg/kg), NEO (neosolaninol; 100 μg/kg), NIV (nivalenol; 100 μg/kg),
ZON (zearalenone; 100 μg/kg).
For clarity only 2 MRM transitions are displayed per compound and the following MRM chromatograms were changed; neosolaniol (x0.3), T2 (x0.3),
aflatoxins (x3), fumonisins (x2).