Page 18 - Application Notebook - Solution for Food Safety
P. 18
Application No.C136
News
n Results and Discussion Expanded capability of the LCMS-8060
Shimadzu Pesticide MRM Library The LCMS-8060 has a data acquisition speed of
(Application News No. C135 ) 30,000 u/sec which creates new opportunities for
expanding compound lists.
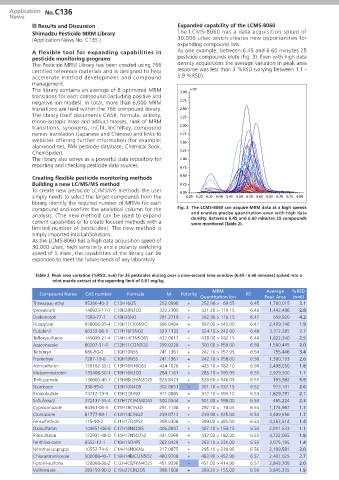
A flexible tool for expanding capabilities in As one example, between 6.45 and 6.60 minutes 25
pesticide monitoring programs pesticide compounds elute (Fig. 3). Even with high data
The Pesticide MRM Library has been created using 766 density acquisitions the average variation in peak area
certified reference materials and is designed to help response was less than 3 %RSD (varying between 1.1 -
accelerate method development and compound 5.9 %RSD).
management.
The library contains an average of 8 optimized MRM 3.00 ×10 6
transitions for each compound (including positive and
negative ion modes). In total, more than 6,000 MRM 2.75
transitions are held within the 766 compound library. 2.50
The library itself documents CAS#, formula, activity, 2.25
mono-isotopic mass and adduct masses, rank of MRM
transitions, synonyms, InChI, InChIKey, compound 2.00
names translation (Japanese and Chinese) and links to 1.75
websites offering further information (for example; 1.50
alanwood.net, PAN pesticide database, Chemical Book,
ChemSpider). 1.25
The library also serves as a powerful data repository for 1.00
reporting and checking pesticide data sources. 0.75
Creating flexible pesticide monitoring methods 0.50
Building a new LC/MS/MS method 0.25
To create new pesticide LC/MS/MS methods the user 0.00
simply needs to select the target compounds from the 6.25 6.30 6.35 6.40 6.45 6.50 6.55 6.60 6.65 6.70 6.75 6.80
library, identify the required number of MRMs for each
compound and confirm the analytical column for the Fig. 3 The LCMS-8060 can acquire MRM data at a high speeds
analysis. (The new method can be used to expand and enables precise quantitation even with high data
current capabilities or to create focused methods with a density. Between 6.45 and 6.60 minutes 25 compounds
were monitored (Table 2).
limited number of pesticides). The new method is
simply imported into LabSolutions.
As the LCMS-8060 has a high data acquisition speed of
30,000 u/sec, high sensitivity and a polarity switching
speed of 5 msec, the capabilities of the library can be
expanded to meet the future needs of any laboratory.
Table 2 Peak area variation (%RSD; n=6) for 25 pesticides eluting over a nine-second time window (6.45 - 6.60 minutes) spiked into a
mint matrix extract at the reporting limit of 0.01 mg/kg.
MRM Average %RSD
Compound Name CAS number Formula M Polarity RT
Quantitation Ion Peak Area (n=6)
Trinexapac-ethyl 95266-40-3 C13H16O5 252.0998 + 252.90 > 69.05 6.45 1,780,015 3.1
Iprovalicarb 140923-17-7 C18H28N2O3 320.2100 + 321.20 > 119.15 6.46 1,442,486 2.8
Dodemorph 1593-77-7 C18H35NO 281.2719 + 282.30 > 116.15 6.47 658,920 4.2
Fluopyram 658066-35-4 C16H11ClF6N2O 396.0464 + 397.00 > 145.00 6.47 2,439,146 1.9
Flutolanil 66332-96-5 C17H16F3NO2 323.1133 + 324.10 > 242.00 6.48 3,372,285 2.7
Trifloxysulfuron 145099-21-4 C14H14F3N5O6S 437.0617 + 438.00 > 182.15 6.48 1,822,340 2.5
Azaconazole 60207-31-0 C12H11Cl2N3O2 299.0228 + 300.00 > 159.00 6.50 1,580,445 2.0
Terbutryn 886-50-0 C10H19N5S 241.1361 + 242.10 > 157.95 6.50 755,446 3.4
Prometryn 7287-19-6 C10H19N5S 241.1361 + 242.10 > 158.00 6.50 1,300,193 2.6
Azimsulfuron 120162-55-2 C13H16N10O5S 424.1026 + 425.10 > 182.10 6.50 2,498,050 1.8
Metominostrobin 133408-50-1 C16H16N2O3 284.1161 + 285.10 > 193.95 6.51 2,929,500 1.7
Thifluzamide 130000-40-7 C13H6Br2F6N2O2S 525.8421 + 528.60 > 148.05 6.51 193,982 5.9
Nicarbazin 330-95-0 C13H10N4O5 302.0651 - 301.10 > 137.15 6.52 973,101 2.6
Bromobutide 74712-19-9 C15H22BrNO 311.0885 + 312.10 > 194.10 6.53 1,829,781 2.1
Saflufenacil 372137-35-4 C17H17ClF4N4O5S 500.0544 + 501.00 > 198.00 6.53 465,224 2.3
Cyproconazole 94361-06-5 C15H18ClN3O 291.1138 + 292.10 > 70.05 6.54 1,174,967 1.7
Clomazone 81777-89-1 C12H14ClNO2 239.0713 + 239.90 > 125.00 6.54 3,409,656 1.7
Fensulfothion 115-90-2 C11H17O4PS2 308.0306 + 309.00 > 281.00 6.54 4,267,514 1.4
Oxasulfuron 144651-06-9 C17H18N4O6S 406.0947 + 407.10 > 150.15 6.54 2,911,533 1.1
Rimsulfuron 122931-48-0 C14H17N5O7S2 431.0569 + 432.00 > 182.00 6.55 4,722,065 1.8
Fenthion-oxon 6552-12-1 C10H15O4PS 262.0429 + 263.10 > 231.00 6.55 3,075,195 1.4
Nitrothal-isopropyl 10552-74-6 C14H16NO6Na 317.0875 + 295.10 > 230.95 6.56 2,199,581 3.0
Chlorantraniliprole 500008-45-7 C18H14BrCl2N5O2 480.9708 + 483.90 > 452.90 6.57 2,407,025 2.7
Fipronil-sulfone 120068-36-2 C12H4Cl2F6N4O2S 451.9336 - 451.00 > 414.90 6.57 2,843,708 2.0
Valifenalate 283159-90-0 C19H27ClN2O5 398.1608 + 399.20 > 155.00 6.59 3,845,335 1.9