Page 17 - Application Notebook - Solution for Food Development
P. 17
Application No.C133
News
ʢ×1,000 ʣ ʢ×100ʣ ʢ ×1,000ʣ ʢ×100ʣ
1.0
2.0
5 ng/mL
0.5 ng/mL 50 ng/mL 50 ng/mL
1.0
2.5
0.5 1.0
0.0 0.0 0.0 0.0
6.5 7.0 7.0 7.5 7.0 7.5 7.5 8.0
"TQBSUBNF "EWBOUBNF (MZDZSSIJ[JD BDJE 3FCBVEJPTJEF .
ʢ×100ʣ ʢ×100ʣ ʢ×1,000 ʣ ʢ ×1,000ʣ
2.5
1 ng/mL 1 ng/mL 10 ng/mL 2.0 0.5 ng/mL
2.5 1.0
1.0
0.0 0.0 0.0
7.5 8.0 8.0 8.5 8.0 8.5 10.0 10.5
/FPUBNF 3FCBVEJPTJEF $ %VMDPTJEF " *TPTUFWJPM
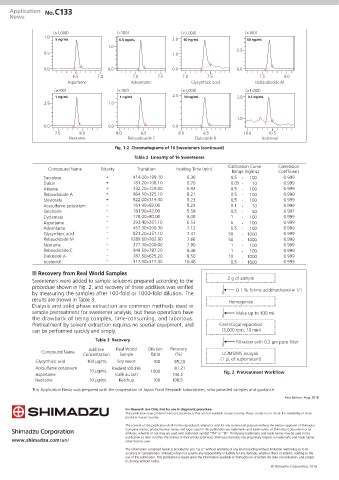
Fig. 1-2 Chromatograms of 16 Sweeteners (continued)
Table 2 Linearity of 16 Sweeteners
Calibration Curve Correlation
Compound Name Polarity Transition Holding Time (min)
Range (ng/mL) Coefficient
Sucralose + 414.00>199.10 6.36 0.5 ‐ 100 0.999
Dulcin + 181.20>108.10 6.70 0.05 ‐ 10 0.999
Alitame + 332.20>129.00 6.92 0.5 ‐ 100 0.999
Rebaudioside A + 984.50>325.10 8.21 0.5 ‐ 100 0.999
Stevioside + 822.00>319.30 8.23 0.5 ‐ 100 0.999
Acesulfame potassium - 161.90>82.00 5.23 0.1 ‐ 10 0.999
Saccharin - 181.90>42.00 5.58 0.5 ‐ 50 0.997
Cyclamate - 178.00>80.00 6.08 1 ‐ 100 0.999
Aspartame - 293.40>261.10 6.53 5 ‐ 100 0.999
Advantame - 457.30>200.30 7.12 0.5 ‐ 100 0.999
Glycyrrhizic acid - 821.20>351.10 7.41 50 ‐ 1000 0.999
Rebaudioside M - 1289.60>802.90 7.66 50 ‐ 1000 0.999
Neotame - 377.30>200.00 7.90 1 ‐ 100 0.999
Rebaudioside C - 949.50>787.20 8.46 1 ‐ 100 0.999
Dulcoside A - 787.50>625.20 8.50 10 ‐ 1000 0.999
Isosteviol - 317.30>317.30 10.46 0.5 ‐ 1000 0.999
Q Recovery from Real World Samples
2 g of sample
Sweeteners were added to sample solutions prepared according to the
procedure shown in Fig. 2, and recovery of these additives was verified 0.1 % formic acid/methanol = 1/1
by measuring the samples after 100-fold or 1000-fold dilution. The
results are shown in Table 3. Homogenize
Dialysis and solid phase extraction are common methods used in
sample pretreatment for sweetener analysis, but these operations have Make up to 100 mL
the drawback of being complex, time-consuming, and laborious.
Pretreatment by solvent extraction requires no special equipment, and Centrifugal separation
can be performed quickly and simply. (3,000 rpm, 10 min)
Table 3 Recovery Filtration with 0.2 μm pore filter
Additive Real World Dilution Recovery
Compound Name
Concentration Sample Ratio (%) LC/MS/MS analysis
(1 μL of supernatant)
Glycyrrhizic acid 100 μg/mL Soy sauce 100 85.20
Acesulfame potassium Powdered soft drink 81.21
10 μg/mL 1000 Fig. 2 Pretreatment Workflow
Aspartame (café au lait) 104.2
Neotame 10 μg/mL Ketchup 100 108.5
This Application News was prepared with the cooperation of Japan Food Research Laboratories, who provided samples and guidance.
First Edition: Aug. 2016
For Research Use Only. Not for use in diagnostic procedure.
This publication may contain references to products that are not available in your country. Please contact us to check the availability of these
products in your country.
The content of this publication shall not be reproduced, altered or sold for any commercial purpose without the written approval of Shimadzu.
Company names, product/service names and logos used in this publication are trademarks and trade names of Shimadzu Corporation or its
affiliates, whether or not they are used with trademark symbol “TM” or “®”. Third-party trademarks and trade names may be used in this
publication to refer to either the entities or their products/services. Shimadzu disclaims any proprietary interest in trademarks and trade names
www.shimadzu.com/an/ other than its own.
The information contained herein is provided to you "as is" without warranty of any kind including without limitation warranties as to its
accuracy or completeness. Shimadzu does not assume any responsibility or liability for any damage, whether direct or indirect, relating to the
use of this publication. This publication is based upon the information available to Shimadzu on or before the date of publication, and subject
to change without notice.
© Shimadzu Corporation, 2016