Page 11 - Pharmaceutical Solution for Pharma Analysis
P. 11
LC/MS/MS MRM Library for Phospholipid Profiling
For LabSolutions Version 5
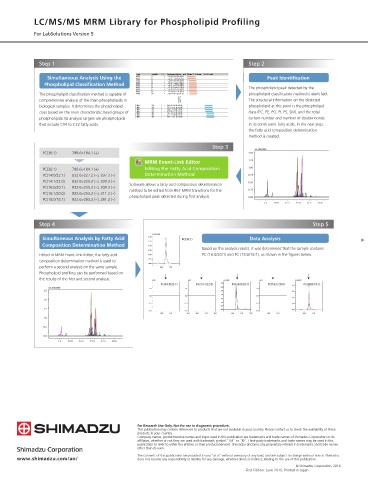
Step 1 Step 2
Simultaneous Analysis Using the Peak Identification
Phospholipid Classification Method
The phospholipid peak detected by the
The phospholipid classification method is capable of phospholipid classification method is identified.
comprehensive analysis of the main phospholipids in The structural information on the detected
biological samples. It determines the phospholipid phospholipid at this point is the phospholipid
class based on the main characteristic head groups of class (PC, PE, PG, PI, PS, SM), and the total
phospholipids. Its analysis targets are phospholipids carbon number and number of double bonds
that include C14 to C22 fatty acids. in its constituent fatty acids. In the next step,
the fatty acid composition determination
method is created.
Step 3
(x1,000,000)
PC(36:1) 788.6>184.1 (+) 1.50
MRM Event-Link Editor 1.25
PC(36:1) 788.6>184.1 (+) Editing the Fatty Acid Composition 1.00
PC(14:0/22:1) 832.6>227.2 (−), 337.3 (−) Determination Method 0.75
PC(14:1/22:0) 832.6>225.2 (−), 339.3 (−) Software allows a fatty acid composition determination 0.50
PC(16:0/20:1) 832.6>255.2 (−), 309.3 (−) method to be edited from 867 MRM transitions for the 0.25
PC(16:1/20:0) 832.6>253.2 (−), 311.3 (−) phospholipid peak detected during first analysis.
PC(18:0/18:1) 832.6>283.3 (−), 281.3 (−) 0.00
7.5 10.0 12.5 15.0 17.5 20.0
Step 4 Step 5
(×10,000,000)
Simultaneous Analysis by Fatty Acid 1.50 PC(36:1) Data Analysis
1.25
Composition Determination Method 1.00
0.75 Based on the analysis results, it was determined that the sample contains
Edited in MRM Event-Link Editor, the fatty acid 0.50 PC (16:0/20:1) and PC (18:0/18:1), as shown in the figures below.
composition determination method is used to 0.25
0.00
perform a second analysis on the same sample. 16.0 17.0
Phospholipid profiling can be performed based on
the results of the first and second analysis. (×10) (×10) (×10,000) (×10) (×100,000)
PC(14:0/22:1) PC(14:1/22:0) 3.5 PC(16:0/20:1) PC(16:1/20:0) 4.0 PC(18:0/18:1)
(x1,000,000) 2.5 2.5 3.0 2.5
2.5 2.5 3.0
2.0
0.0 0.0 0.0
2.0 1.5 2.0
-2.5 -2.5 1.0 -2.5 1.0
0.5
1.5 0.0 0.0
-5.0 -5.0 -5.0
16.0 17.0 15.0 16.0 17.0 18.0 16.0 17.0 18.0 16.0 17.0 16.0 17.0
1.0
0.5
0.0
7.5 10.0 12.5 15.0 17.5 20.0
For Research Use Only. Not for use in diagnostic procedure.
This publication may contain references to products that are not available in your country. Please contact us to check the availability of these
products in your country.
Company names, product/service names and logos used in this publication are trademarks and trade names of Shimadzu Corporation or its
affiliates, whether or not they are used with trademark symbol “TM” or “®”. Third-party trademarks and trade names may be used in this
publication to refer to either the entities or their products/services. Shimadzu disclaims any proprietary interest in trademarks and trade names
other than its own.
The contents of this publication are provided to you “as is” without warranty of any kind, and are subject to change without notice. Shimadzu
www.shimadzu.com/an/ does not assume any responsibility or liability for any damage, whether direct or indirect, relating to the use of this publication.
© Shimadzu Corporation, 2016
First Edition: June 2016, Printed in Japan