Page 13 - Pharmaceutical Solution for Pharma Analysis
P. 13
Application No. B61
News
(x1,000,000)
7.5 646.037
5.0
2.5
618.005
0.0
600 610 620 630 640 650 660 670 680 690 700
m/z
Mass Spectrum of Tissue Section
(Detail of m/z 600 to 700 Range)
Section of lung tissue with administered
(stained with HE)
MS Image (Corresponding to Molecular Weight of Metabolite)
Section of lung tissue with amiodarone administered
Slide of unstained histological
(Unstained for MS imaging analysis)
section
Matrix coating
MS imaging analysis
Matrix removed
HE staining
Slide of HE-stained histological
section
MS image (unchanged amiodarone)
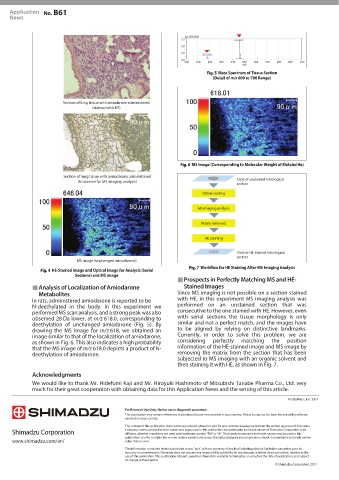
HE-Stained Image and Optical Image for Analysis (Serial Workflow for HE Staining After MS Imaging Analysis
Sections) and MS Image
Prospects in Perfectly Matching MS and HE-
Analysis of Localization of Amiodarone Stained Images
Metabolites Since MS imaging is not possible on a section stained
In rats, administered amiodarone is reported to be with HE, in this experiment MS imaging analysis was
N-deethylated in the body. In this experiment we performed on an unstained section that was
performed MS scan analysis, and a strong peak was also consecutive to the one stained with HE. However, even
observed 28 Da lower, at m/z 618.0, corresponding to with serial sections the tissue morphology is only
deethylation of unchanged amiodarone (Fig. 5). By similar and not a perfect match, and the images have
drawing the MS image for m/z 618, we obtained an to be aligned by relying on distinctive landmarks.
image similar to that of the localization of amiodarone, Currently, in order to solve this problem, we are
as shown in Fig. 6. This also indicates a high probability considering perfectly matching the position
that the MS image of m/z 618.0 depicts a product of N- information of the HE-stained image and MS image by
deethylation of amiodarone. removing the matrix from the section that has been
subjected to MS imaging with an organic solvent and
then staining it with HE, as shown in Fig. 7.
Acknowledgments
We would like to thank Mr. Hidefumi Kaji and Mr. Hiroyuki Hashimoto of Mitsubishi Tanabe Pharma Co., Ltd. very
much for their great cooperation with obtaining data for this Application News and the writing of this article.
First Edition: Jun. 2017
For Research Use Only. Not for use in diagnostic procedure.
This publication may contain references to products that are not available in your country. Please contact us to check the availability of these
products in your country.
The content of this publication shall not be reproduced, altered or sold for any commercial purpose without the written approval of Shimadzu.
Company names, product/service names and logos used in this publication are trademarks and trade names of Shimadzu Corporation or its
affiliates, whether or not they are used with trademark symbol “TM” or “£”. Third-party trademarks and trade names may be used in this
publication to refer to either the entities or their products/services. Shimadzu disclaims any proprietary interest in trademarks and trade names
www.shimadzu.com/an/ other than its own.
The information contained herein is provided to you "as is" without warranty of any kind including without limitation warranties as to its
accuracy or completeness. Shimadzu does not assume any responsibility or liability for any damage, whether direct or indirect, relating to the
use of this publication. This publication is based upon the information available to Shimadzu on or before the date of publication, and subject
to change without notice.
© Shimadzu Corporation, 2017