Page 17 - Pharmaceutical Solution for Pharma Analysis
P. 17
Application No.L464
News
Table 3 Analytical Conditions Table 5 Analytical Conditions
System : (1) Prominence System : (1) Prominence
(2) Nexera X2 (Loop injection with 20 µL loop) (2) Nexera X2 (Loop injection with 20 µL loop)
Column : (1) Shim-pack VP-ODS (150 mm L. × 4.6 mm I.D., 4.6 µm) Column : Shim-pack VP-ODS (150 mm L. × 4.6 mm I.D., 4.6 µm)
(2) Shim-pack XR-ODS Ⅲ (50 mm L. × 2.0 mm I.D., 1.6 µm) Mobile Phase : Sodium phosphate buffer <pH 2.8> / methanol = 65/35 (v/v)
Mobile Phase : Methanol / water / acetic acid = 10/89/1 (v/v/v) Column Temp. : 40 °C
Column Temp. : Ambient Flowrate : 1.2 mL/min
Flowrate (1) 0.8 mL/min Injection Vol. : 10 µL
(2) 0.47 mL/min Detection : (1) SPD-20AV at 295 nm
Injection Vol. : (1) 10 µL (2) SPD-M30A at 295 nm
(2) 2 µL Flow Cell : (1) Conventional cell (for SPD-20A(V))
Detection : (1) SPD-20AV at 254 nm (2) Standard cell (for SPD-M30A)
(2) SPD-M30A at 254 nm
Flow Cell : (1) Conventional cell (for SPD-20A(V))
(2) Standard cell (for SPD-M30A)
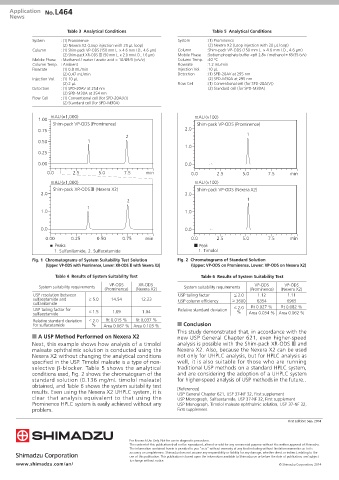
mAU (×1,000) mAU (×100)
1.00
Shim-pack VP-ODS (Prominence) Shim-pack VP-ODS (Prominence)
0.75 2.0
2 1
0.50 1
1.0
0.25
0.00 0.0
0.0 2.5 5.0 7.5 min 0.0 2.5 5.0 7.5 min
mAU (×1,000) mAU (×100)
Shim-pack XR-ODSᶙ (Nexera X2) Shim-pack VP-ODS (Nexera X2)
2.0 2.0
2 1
1
1.0 1.0
0.0 0.0
0.00 0.25 0.50 0.75 min 0.0 2.5 5.0 7.5 min
■ Peaks ■ Peak
1. Sulfanilamide, 2. Sulfacetamide 1. Timolol
Fig. 1 Chromatograms of System Suitability Test Solution Fig. 2 Chromatograms of Standard Solution
(Upper: VP-ODS with Prominence, Lower: XR-ODS Ⅲ with Nexera X2) (Upper: VP-ODS on Prominence, Lower: VP-ODS on Nexera X2)
Table 4 Results of System Suitability Test Table 6 Results of System Suitability Test
VP-ODS XR-ODSⅢ VP-ODS VP-ODS
System suitability requirements (Prominence) (Nexera X2) System suitability requirements (Prominence) (Nexera X2)
USP resolution between USP tailing factor ≦ 2.0 1.12 1.11
sulfacetamide and ≧ 5.0 14.54 12.23 USP column efficiency ≧ 3600 6354 6965
sulfanilamide
USP tailing factor for ≦ 1.5 1.09 1.04 Relative standard deviation ≦ 2.0 Rt 0.027 % Rt 0.082 %
sulfacetamide % Area 0.034 % Area 0.062 %
Relative standard deviation ≦ 2.0 Rt 0.015 % Rt 0.037 %
for sulfacetamide % Area 0.067 % Area 0.103 % n Conclusion
This study demonstrated that, in accordance with the
n A USP Method Performed on Nexera X2 new USP General Chapter 621, even higher-speed
Next, this example shows how analysis of a timolol analysis is possible with the Shim-pack XR-ODS Ⅲ and
maleate ophthalmic solution is conducted using the Nexera X2. Also, because the Nexera X2 can be used
Nexera X2 without changing the analytical conditions not only for UHPLC analysis, but for HPLC analysis as
specified in the USP. Timolol maleate is a type of non- well, it is also suitable for those who are running
selective β-blocker. Table 5 shows the analytical traditional USP methods on a standard HPLC system,
conditions used, Fig. 2 shows the chromatogram of the and are considering the adoption of a UHPLC system
standard solution (0.136 mg/mL timolol maleate) for higher-speed analysis of USP methods in the future..
obtained, and Table 6 shows the system suitability test
results. Even using the Nexera X2 UHPLC system, it is [References]
USP General Chapter 621, USP 37-NF 32, First supplement
clear that analysis equivalent to that using the USP Monograph, Sulfacetamide, USP 37-NF 32, First supplement
Prominence HPLC system is easily achieved without any USP Monograph, Timolol maleate ophthalmic solution, USP 37-NF 32,
problem. First supplement
First Edition: Sep. 2014
For Research Use Only. Not for use in diagnostic procedures.
The content of this publication shall not be reproduced, altered or sold for any commercial purpose without the written approval of Shimadzu.
The information contained herein is provided to you "as is" without warranty of any kind including without limitation warranties as to its
accuracy or completeness. Shimadzu does not assume any responsibility or liability for any damage, whether direct or indirect, relating to the
use of this publication. This publication is based upon the information available to Shimadzu on or before the date of publication, and subject
to change without notice.
www.shimadzu.com/an/ © Shimadzu Corporation, 2014