Page 20 - Pharmaceutical Solution for Pharma Analysis
P. 20
LAAN-A-LC-E292
Application High Performance Liquid Chromatography
News
High Speed Analysis of Pharmaceutical Impurities in
Compliance with European Pharmacopoeia Using
No. L518 Nexera-i MT
In recent years, the development of short-time analytical In this research we examined reducing the analysis
methods for improving analytical task efficiency and time within the adjustment range allowed by the EP.
productivity is promoting the uptake of an ultra-high- Table 1 lists the analytical conditions that comply with
speed analytical technology that uses UHPLC systems both the ivermectin related substances testing
*2
and columns packed with microparticles in research and section and the allowable adjustment range assigned
development departments in the pharmaceutical field. in the EP. Since the Nexera-i MT used in analysis
This trend also applies to pharmacopoeia. For example, features both HPLC and UHPLC flow lines, it allows
according to "Adjustment of chromatographic migration between conventional analysis and high
*1
condition" described in the 8th edition of the European speed analysis within a single system. The Shim-pack
Pharmacopoeia (EP), adjustments to parameters in TLC, GIST C18 series was used for the analytical columns.
LC, GC and SFC are only allowed when the system The analytical conditions other than the analytical
suitability requirements are satisfied. In such a case, columns and flow rate are the same as those listed in
revalidation is not required. the EP.
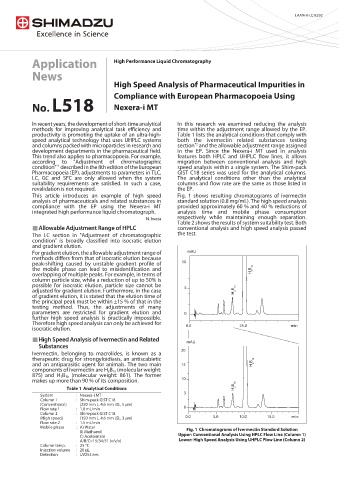
This article introduces an example of high speed Fig. 1 shows resulting chromatograms of ivermectin
analysis of pharmaceuticals and related substances in standard solution (0.8 mg/mL). The high speed analysis
compliance with the EP using the Nexera-i MT provided approximately 60 % and 40 % reductions of
integrated high performance liquid chromatograph. analysis time and mobile phase consumption
respectively while maintaining enough separation.
N. Iwata
Table 2 shows the results of system suitability test. Both
Allowable Adjustment Range of HPLC conventional analysis and high speed analysis passed
The LC section in "Adjustment of chromatographic the test.
condition" is broadly classified into isocratic elution
and gradient elution.
For gradient elution, the allowable adjustment range of mAU
methods differs from that of isocratic elution because
peak-shifting caused by unstable gradient profile of 10
the mobile phase can lead to misidentification and H 2 B 1a
overlapping of multiple peaks. For example, in terms of
column particle size, while a reduction of up to 50% is
possible for isocratic elution, particle size cannot be 5
adjusted for gradient elution. Furthermore, in the case H 2 B 1b
of gradient elution, it is stated that the elution time of
the principal peak must be within r15 % of that in the
testing method. Thus, the adjustments of many
parameters are restricted for gradient elution and 0
further high speed analysis is practically impossible.
Therefore high speed analysis can only be achieved for 0.0 25.0 min
isocratic elution.
High Speed Analysis of Ivermectin and Related mAU
Substances
20
Ivermectin, belonging to macrolides, is known as a
therapeutic drug for strongyloidiasis, an antiscabietic H 2 B 1a
and an antiparasitic agent for animals. The two main 15
components of ivermectin are H 2B 1a (molecular weight:
875) and H 2B 1b (molecular weight: 861). The former
makes up more than 90 % of its composition. 10
Table 1 Analytical Conditions H 2 B 1b
5
System : Nexera-i MT
Column 1 : Shim-pack GIST C18
(Conventional) (250 mm L, 4.6 mm I.D., 5 μm) 0
Flow rate 1 : 1.0 mL/min
Column 2 : Shim-pack GIST C18 10.0 15.0
(High speed) (150 mm L, 4.6 mm I.D., 3 μm) 0.0 5.0 min
Flow rate 2 : 1.5 mL/min
Mobile phase : A) Water Chromatograms of Ivermectin Standard Solution
B) Methanol
C) Acetonitrile Upper: Conventional Analysis Using HPLC Flow Line (Column 1)
A/B/C=15/34/51 (v/v/v) Lower: High Speed Analysis Using UHPLC Flow Line (Column 2)
Column temp. : 25 qC
Injection volume : 20 μL
Detection : UV254 nm