Page 25 - Pharmaceutical Solution for Pharma Analysis
P. 25
LC-15-ADI-036
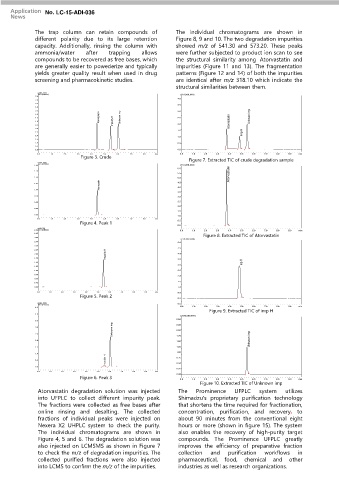
The trap column can retain compounds of The individual chromatograms are shown in
different polarity due to its large retention Figure 8, 9 and 10. The two degradation impurities
capacity. Additionally, rinsing the column with showed m/z of 541.30 and 573.20. These peaks
ammonia/water after trapping allows were further subjected to product ion scan to see
compounds to be recovered as free bases, which the structural similarity among Atorvastatin and
are generally easier to powederize and typically impurities (Figure 11 and 13). The fragmentation
yields greater quality result when used in drug patterns (Figure 12 and 14) of both the impurities
screening and pharmacokinetic studies. are identical after m/z 318.10 which indicate the
structural similarities between them.
mV(x1,000)
Ch1 (245nm) (x10,000,000)
7.5
4.0
7.0
6.5
3.5
6.0
5.5 3.0
5.0
4.5 Atorvastatin Unknown imp 2.5 Unkwon imp
4.0 Impurity H Atorvastatin
3.5 2.0
3.0
1.5
2.5 Imp H
2.0
1.0
1.5
1.0 0.5
0.5
0.0 0.0
0.0 1.0 2.0 3.0 4.0 5.0 6.0 7.0 8.0 min 0.0 1.0 2.0 3.0 4.0 5.0 6.0 7.0 8.0 9.0 min
Figure 3. Crude
Figure 7. Extracted TIC of crude degradation sample
mV(x1,000)
Ch1 (245nm)
2.00 (x10,000,000)
6.0
1.75
5.5 Atorvastatin
1.50 5.0
Atorvastatin 4.0
1.25 4.5
1.00
3.5
0.75 3.0
0.50 2.5
2.0
0.25
1.5
0.00 1.0
0.0 1.0 2.0 3.0 4.0 5.0 6.0 7.0 8.0 min 0.5
Figure 4. Peak 1
0.0
mV(x100)
3.50 Ch1 (245nm) 0.0 1.0 2.0 3.0 4.0 5.0 6.0 7.0 8.0 9.0 min
3.25
Figure 8. Extracted TIC of Atorvastatin
3.00
(x10,000,000)
2.75 5.0
2.50
4.5
2.25
2.00 Impurity H 4.0
1.75 3.5
1.50 Imp H
3.0
1.25
1.00 2.5
0.75
2.0
0.50
1.5
0.25
0.00 1.0
0.0 1.0 2.0 3.0 4.0 5.0 6.0 7.0 8.0 9.0 min 0.5
Figure 5. Peak 2
0.0
mV(x1,000) -0.5
Ch1 (245nm)
4.5 0.0 1.0 2.0 3.0 4.0 5.0 6.0 7.0 8.0 9.0 min
Figure 9. Extracted TIC of imp H
4.0
(x100,000,000)
2.50
3.5
Unknown imp 2.00
3.0 2.25
2.5 1.75
2.0 1.50 Unkwon imp
1.5 1.25
1.00
1.0
0.5 Impurity H 0.75
0.50
0.0
0.25
0.0 1.0 2.0 3.0 4.0 5.0 6.0 7.0 8.0 9.0 min
0.00
Figure 6. Peak 3 0.0 1.0 2.0 3.0 4.0 5.0 6.0 7.0 8.0 9.0 min
Figure 10. Extracted TIC of Unknown imp
Atorvastatin degradation solution was injected The Prominence UFPLC system utilizes
into UFPLC to collect different impurity peak. Shimadzu's proprietary purification technology
The fractions were collected as free bases after that shortens the time required for fractionation,
online rinsing and desalting. The collected concentration, purification, and recovery, to
fractions of individual peaks were injected on about 90 minutes from the conventional eight
Nexera X2 UHPLC system to check the purity. hours or more (shown in figure 15). The system
The individual chromatograms are shown in also enables the recovery of high-purity target
Figure 4, 5 and 6. The degradation solution was compounds. The Prominence UFPLC greatly
also injected on LCMSMS as shown in Figure 7 improves the efficiency of preparative fraction
to check the m/z of degradation impurities. The collection and purification workflows in
collected purified fractions were also injected pharmaceutical, food, chemical and other
into LCMS to confirm the m/z of the impurities. industries as well as research organizations.