Page 21 - Pharmaceutical Solution for Pharma Analysis
P. 21
Application No. L518
News
Table 2 Results of System Suitability Test
mAU
System suitability Results Judgements
requirements Conventional High speed
10
Resolution
(H2B1b and H2B1a) ≥ 3.0 5.1 4.7 PASS Diclofenac
Signal-to-noise ratio ≥ 10 40 38 PASS
(0.4 μg/mL) F
5
Symmetry factor ≤ 2.5 1.1 1.2 PASS
A
High Speed Analysis of Diclofenac Sodium and
Related Substances 0
Diclofenac is widely used as an antipyretic and a pain- 0.0 25.0 50.0 min
reliever. Here we introduce an example of high speed
analysis of a diclofenac sodium and related substances
based on the EP. mAU
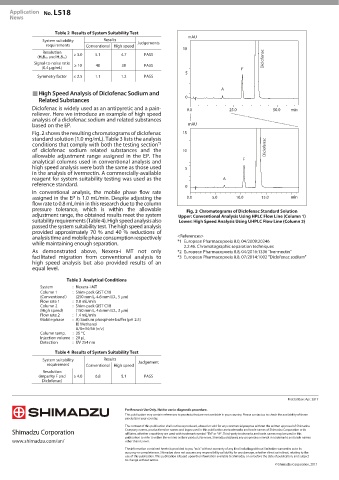
Fig. 2 shows the resulting chromatograms of diclofenac 15
standard solution (1.0 mg/mL). Table 3 lists the analysis
*3
conditions that comply with both the testing section Diclofenac
of diclofenac sodium related substances and the 10
allowable adjustment range assigned in the EP. The F
analytical columns used in conventional analysis and
high speed analysis were both the same as those used 5
in the analysis of ivermectin. A commercially-available
reagent for system suitability testing was used as the A
reference standard. 0
In conventional analysis, the mobile phase flow rate
assigned in the EP is 1.0 mL/min. Despite adjusting the 0.0 5.0 10.0 15.0 min
flow rate to 0.8 mL/min in this research due to the column
pressure tolerance, which is within the allowable Chromatograms of Diclofenac Standard Solution
adjustment range, the obtained results meet the system Upper: Conventional Analysis Using HPLC Flow Line (Column 1)
suitability requirements (Table 4). High speed analysis also Lower: High Speed Analysis Using UHPLC Flow Line (Column 2)
passed the system suitability test. The high speed analysis
provided approximately 70 % and 40 % reductions of
analysis time and mobile phase consumption respectively <References>
while maintaining enough separation. *1 European Pharmacopoeia 8.0, 04/2009:20246
2.2.46. Chromatographic separation techniques
As demonstrated above, Nexera-i MT not only *2 European Pharmacopoeia 8.8, 04/2016:1336 "Ivermectin"
facilitated migration from conventional analysis to *3 European Pharmacopoeia 8.8, 07/2014:1002 "Diclofenac sodium"
high speed analysis but also provided results of an
equal level.
Table 3 Analytical Conditions
System : Nexera-i MT
Column 1 : Shim-pack GIST C18
(Conventional) (250 mm L, 4.6 mm I.D., 5 Pm)
Flow rate 1 : 0.8 mL/min
Column 2 : Shim-pack GIST C18
(High speed) (150 mm L, 4.6 mm I.D., 3 Pm)
Flow rate 2 : 1.4 mL/min
Mobile phase : A) Sodium phosphate buffer (pH 2.5)
B) Methanol
A/B=34/66 (v/v)
Column temp. : 25 qC
Injection volume : 20 μL
Detection : UV 254 nm
Table 4 Results of System Suitability Test
System suitability Results Judgement
requirement Conventional High speed
Resolution
(impurity F and ≥ 4.0 6.8 5.1 PASS
Diclofenac)
First Edition: Apr. 2017
For Research Use Only. Not for use in diagnostic procedure.
This publication may contain references to products that are not available in your country. Please contact us to check the availability of these
products in your country.
The content of this publication shall not be reproduced, altered or sold for any commercial purpose without the written approval of Shimadzu.
Company names, product/service names and logos used in this publication are trademarks and trade names of Shimadzu Corporation or its
affiliates, whether or not they are used with trademark symbol “TM” or “£”. Third-party trademarks and trade names may be used in this
publication to refer to either the entities or their products/services. Shimadzu disclaims any proprietary interest in trademarks and trade names
www.shimadzu.com/an/ other than its own.
The information contained herein is provided to you "as is" without warranty of any kind including without limitation warranties as to its
accuracy or completeness. Shimadzu does not assume any responsibility or liability for any damage, whether direct or indirect, relating to the
use of this publication. This publication is based upon the information available to Shimadzu on or before the date of publication, and subject
to change without notice.
© Shimadzu Corporation, 2017