Page 26 - Pharmaceutical Solution for Pharma Analysis
P. 26
LC-15-ADI-036
(x100,000,000) 150 Inten.
1.0
140
0.9 130
0.8 120
110 422.15
0.7
100 448.15
0.6 90
80
0.5
70
0.4 60
0.3 50
40
0.2 30
0.1 20 250.15 276.15 292.10 318.10 362.15 370.05 380.10 406.05 430.20 541.20
10 302.00
0.0
0
1.0 2.0 3.0 4.0 5.0 6.0 7.0 8.0 9.0 min 225.0 250.0 275.0 300.0 325.0 350.0 375.0 400.0 425.0 450.0 475.0 500.0 525.0 m/z
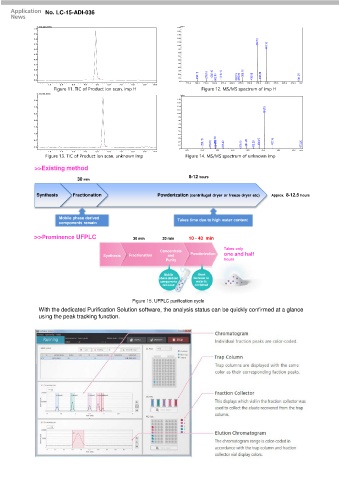
Figure 11. TIC of Product ion scan, imp H Figure 12. MS/MS spectrum of imp H
(x10,000,000)
Inten.
140
3.5
130
3.0 120
110 454.15
2.5 100
90
2.0 80
70
1.5 60
50
1.0
40
30
0.5 292.10
20 250.10 394.20 436.15 480.15
10 276.15 294.10 318.20 376.15 418.20 573.20
0.0
0 200 250 300 350 400 450 500 550 m/z
1.0 2.0 3.0 4.0 5.0 6.0 7.0 8.0 9.0 min
Figure 13. TIC of Product ion scan, unknown imp Figure 14. MS/MS spectrum of unknown imp
>>Existing method
8-12 hours
30 min
Synthesis Fractionation Powderization (centrifugal dryer or freeze dryer etc) Approx. 8-12.5 hours
Mobile phase derived Takes time due to high water content
components remain
>>Prominence UFPLC 30 min 20 min 10 - 40 min
Takes only
Concentrate
Synthesis Fractionation and Powderization one and half
Purify hours
Mobile Short
phase derived because no
components water is
removed contained
Figure 15. UFPLC purification cycle
With the dedicated Purification Solution software, the analysis status can be quickly confirmed at a glance
using the peak tracking function.