Page 31 - Pharmaceutical Solution for Pharma Analysis
P. 31
Application No. L517
News
(× 10,000) (× 1,000)
1:346.10 > 198.10 (+) 1:359.90 > 150.10 (+)
3.0 5.0
(B)
2.5 (A) 4.0 (A)
(B)
2.0
3.0
1.5
2.0
1.0
0.5 1.0
0
0
0 1.0 2.0 3.0 4.0 5.0 0 1.0 2.0 3.0 4.0 5.0
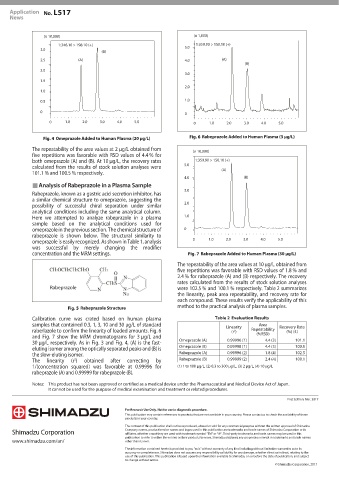
Rabeprazole Added to Human Plasma (3 μg/L)
Omeprazole Added to Human Plasma (20 μg/L)
The repeatability of the area values at 2 μg/L obtained from (× 10,000)
five repetitions was favorable with RSD values of 4.4 % for
both omeprazole (A) and (B). At 10 μg/L, the recovery rates 5.0 1:359.90 > 150.10 (+)
calculated from the results of stock solution analyses were
(A)
101.1 % and 100.5 % respectively.
4.0 (B)
Analysis of Rabeprazole in a Plasma Sample
3.0
Rabeprazole, known as a gastric acid secretion inhibitor, has
a similar chemical structure to omeprazole, suggesting the
possibility of successful chiral separation under similar 2.0
analytical conditions including the same analytical column.
Here we attempted to analyze rabeprazole in a plasma 1.0
sample based on the analytical conditions used for
omeprazole in the previous section. The chemical structure of 0
rabeprazole is shown below. The structural similarity to
0 1.0 2.0 3.0 4.0 5.0
omeprazole is easily recognized. As shown in Table 1, analysis
was successful by merely changing the modifier
concentration and the MRM settings. Rabeprazole Added to Human Plasma (30 μg/L)
The repeatability of the area values at 10 μg/L obtained from
five repetitions was favorable with RSD values of 1.8 % and
2.4 % for rabeprazole (A) and (B) respectively. The recovery
rates calculated from the results of stock solution analyses
Rabeprazole were 102.5 % and 100.1 % respectively. Table 2 summarizes
the linearity, peak area repeatability, and recovery rate for
each compound. These results verify the applicability of this
method to the practical analysis of plasma samples.
Rabeprazole Structure
Calibration curve was crated based on human plasma Table 2 Evaluation Results
samples that contained 0.3, 1, 3, 10 and 30 μg/L of standard Linearity Area Recovery Rate
raberlazole to confirm the linearity of loaded amounts. Fig. 6 (r ) Repeatability (%) (4)
2
(%RSD)
and Fig. 7 show the MRM chromatograms for 3 μg/L and Omeprazole (A) 0.99996 (1) 4.4 (3) 101.1
30 μg/L respectively. As in Fig. 3 and Fig. 4, (A) is the fast-
Omeprazole (B) 0.99998 (1) 4.4 (3) 100.5
eluting isomer among the optically separated peaks and (B) is
the slow-eluting isomer. Rabeprazole (A) 0.99996 (2) 1.8 (4) 102.5
2
The linearity (r ) obtained after correcting by Rabeprazole (B) 0.99999 (2) 2.4 (4) 100.1
1/(concentration squared) was favorable at 0.99996 for (1) 1 to 100 μg/L, (2) 0.3 to 300 μg/L, (3) 2 μg/L, (4) 10 μg/L
rabeprazole (A) and 0.99999 for rabeprazole (B).
Notes: This product has not been approved or certified as a medical device under the Pharmaceutical and Medical Device Act of Japan.
It cannot be used for the purpose of medical examination and treatment or related procedures.
First Edition: Mar. 2017
For Research Use Only. Not for use in diagnostic procedure.
This publication may contain references to products that are not available in your country. Please contact us to check the availability of these
products in your country.
The content of this publication shall not be reproduced, altered or sold for any commercial purpose without the written approval of Shimadzu.
Company names, product/service names and logos used in this publication are trademarks and trade names of Shimadzu Corporation or its
affiliates, whether or not they are used with trademark symbol “TM” or “£”. Third-party trademarks and trade names may be used in this
publication to refer to either the entities or their products/services. Shimadzu disclaims any proprietary interest in trademarks and trade names
www.shimadzu.com/an/ other than its own.
The information contained herein is provided to you "as is" without warranty of any kind including without limitation warranties as to its
accuracy or completeness. Shimadzu does not assume any responsibility or liability for any damage, whether direct or indirect, relating to the
use of this publication. This publication is based upon the information available to Shimadzu on or before the date of publication, and subject
to change without notice.
© Shimadzu Corporation, 2017