Page 29 - Pharmaceutical Solution for Pharma Analysis
P. 29
Application No.L495
News
n Automated Optimization of Chiral Separation ranking results from the optional software. The software
Parameters for Omeprazole automatically ranks all the chromatograms with
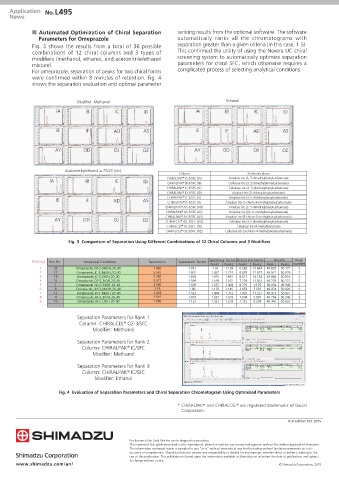
Fig. 3 shows the results from a total of 36 possible separation greater than a given criteria (in this case, 1.5).
combinations of 12 chiral columns and 3 types of This confirmed the utility of using the Nexera UC chiral
modifiers (methanol, ethanol, and acetonitrile/ethanol screening system to automatically optimize separation
mixture). parameters for chiral SFC, which otherwise requires a
For omeprazole, separation of peaks for two chiral forms complicated process of selecting analytical conditions.
were confirmed within 8 minutes of retention. Fig. 4
shows the separation evaluation and optimal parameter
Modifier: Methanol Ethanol
IA IB IC ID IA IB IC ID
IE IF AD AS IE IF AD AS
AY OD OJ OZ AY OD OJ OZ
Acetonitrile/ethanol = 75/25 (v/v)
Column Stationary phase
Amylose tris (3, 5-dimethylphenylcarbamate)
IA IB IC ID CHIRALPAK ® IA-3/SFC (IA) Cellulose tris (3, 5-dimethylphenylcarbamate)
CHIRALPAK ® IB-3/SFC (IB)
CHIRALPAK ® IC-3/SFC (IC) Cellulose tris (3, 5-dichlorophenylcarbamate)
CHIRALPAK ® ID-3/SFC (ID) Amylose tris (3-chlorophenylcarbamate)
CHIRALPAK ® IE-3/SFC (IE) Amylose tris (3, 5-dichlorophenylcarbamate)
IE IF AD AS CHIRALPAK ® IF-3/SFC (IF) Amylose tris (3-chloro-4-methylphenylcarbamate)
CHIRALPAK ® AD-3/SFC (AD) Amylose tris (3, 5-dimethylphenylcarbamate)
CHIRALPAK ® AS-3/SFC (AS) Amylose tris [(S)-Ћ-methylbenzylcarbamate]
CHIRALPAK ® AY-3/SFC (AY) Amylose tris (5-chloro-2-methylphenylcarbamate)
AY OD OJ OZ CHIRALCEL ® OD-3/SFC (OD) Cellulose tris (3,5-dimethylphenylcarbamate)
CHIRALCEL ® OJ-3/SFC (OJ) Cellulose tris (4-methylbenzoate)
CHIRALCEL ® OZ-3/SFC (OZ) Cellulose tris (3-chloro-4-methylphenylcarbamate)
Fig. 3 Comparison of Separation Using Different Combinations of 12 Chiral Columns and 3 Modifiers
Ranking Run No. Analytical Condition Resolution Separatoin factor Symmetry factor Retention factor Area% Peak
Peak1 Peak2 Peak1 Peak2 Peak1 Peak2 number
1 32 Omeprazole_OZ-3_MeOH_20_40 7.965 1.921 1.16 1.159 6.583 12.644 49.829 50.171 2
2 17 Omeprazole_IC-3_MeOH_20_40 5.587 1.602 1.387 1.274 8.078 12.937 49.971 50.029 2
3 16 Omeprazole_IC-3_EtOH_20_40 5.382 1.639 1.915 1.661 8.617 14.124 49.984 50.016 2
4 31 Omeprazole_OZ-3_EtOH_20_40 5.377 1.599 1.169 1.162 7.229 11.561 49.778 50.222 2
5 1 Omeprazole_AD-3_EtOH_20_40 3.996 1.509 1.257 1.404 8.779 13.25 50.054 49.946 2
6 8 Omeprazole_AY-3_MeOH_20_40 3.55 2.08 1.178 1.145 3.652 7.597 49.974 50.026 2
7 11 Omeprazole_IA-3_MeOH_20_40 3.428 1.523 1.464 1.312 7.435 11.327 49.973 50.027 2
8 4 Omeprazole_AS-3_EtOH_20_40 2.515 1.673 1.657 1.518 1.244 2.081 49.754 50.246 2
9 10 Omeprazole_IA-3_EtOH_20_40 1.586 1.157 1.322 1.279 7.115 8.234 49.347 50.653 2
Separation Parameters for Rank 1
Column: CHIRALCEL OZ-3/SFC
®
Modifier: Methanol
Separation Parameters for Rank 2
®
Column: CHIRALPAK IC/SFC
Modifier: Methanol
Separation Parameters for Rank 3
Column: CHIRALPAK IC/SFC
®
Modifier: Ethanol
Fig. 4 Evaluation of Separation Parameters and Chiral Separation Chromatogram Using Optimized Parameters
* CHIRALPAK and CHIRALCEL are registered trademarks of Daicel
®
®
Corporation.
First Edition: Oct. 2015
For Research Use Only. Not for use in diagnostic procedures.
The content of this publication shall not be reproduced, altered or sold for any commercial purpose without the written approval of Shimadzu.
The information contained herein is provided to you "as is" without warranty of any kind including without limitation warranties as to its
accuracy or completeness. Shimadzu does not assume any responsibility or liability for any damage, whether direct or indirect, relating to the
use of this publication. This publication is based upon the information available to Shimadzu on or before the date of publication, and subject
to change without notice.
www.shimadzu.com/an/ © Shimadzu Corporation, 2015