Page 33 - Pharmaceutical Solution for Pharma Analysis
P. 33
Application No. L519
News
Next, by employing the microdialysis method in which biological Table 4 Online SFE-SFC-MS/MS Conditions
compounds are continuously sampled from an awake animal via Vessel : 0.2 mL (1 μL of sample was added to filter paper)
the semipermeable membrane of a minute dialytic probe Extractant : A) Supercritical fluid of CO2
connected to a pump, cerebrospinal fluid was sampled from a rat B) Methanol containing 20 mmol/L ammonium
and directly delivered to SFC analysis. The injection volume of formate / water = 95/5 (v/v)
A/B = 9/1 (v/v)
cerebrospinal fluid was set to 1 μL due to concerns regarding the Flow rate : 2.5 mL/min
miscibility between the aqueous sample and low polar Extraction time : Static (0-3 min) – Dynamic (3-6 min) –
supercritical carbon dioxide, which is the main component of the Static (6-8 min) - Dynamic (8-11 min) –
mobile phase used in SFC. With respect to acetylcholine, the LOQ Static (11-13 min) – Dynamic (13-16 min)
determined according to the ASTM method was about 10 μg/L. BPR pressure : 10 Mpa
Since the calculated concentration was less than the LOQ, only Extraction temp. : 60 °C
: B Conc. 10 % (16 min) → 25 % (26 min) →
Time program
peak identification was performed. As shown in Table 3, the 50 % (26.1-28 min) → 10 % (28.1-31 min)
retention time and peak area repeatabilities were favorable for * SFC-MS/MS conditions are identical to Table 1 except for the time program.
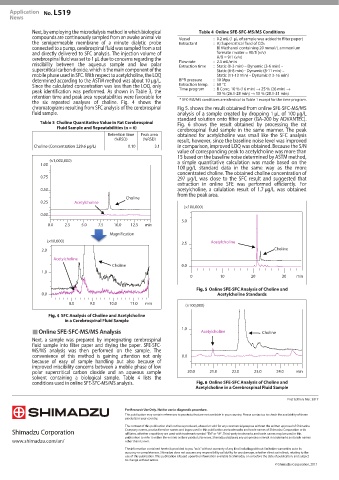
the six repeated analyses of choline. Fig. 4 shows the
chromatograms resulting from SFC analysis of the cerebrospinal Fig 5. shows the result obtained from online SFE-SFC-MS/MS
fluid sample. analysis of a sample created by dropping 1 μL of 100 μg/L
standard solution onto filter paper (GA-200 by ADVANTEC).
Table 3 Choline Quantitative Value in Rat Cerebrospinal Fig. 6 shows the result obtained by processing the rat
Fluid Sample and Repeatabilities (n = 6)
cerebrospinal fluid sample in the same manner. The peak
Retention time Peak area obtained for acetylcholine was small like the SFC analysis
(%RSD) (%RSD)
result, however, since the baseline noise level was improved
Choline (Concentration 229.6 μg/L) 0.10 3.1 in comparison, improved LOQ was obtained. Because the S/N
value of corresponding peak to acetylcholine was more than
15 based on the baseline noise determined by ASTM method,
(×1,000,000)
1.00 a simple quantitative calculation was made based on the
100 μg/L standard data in the same way as the more
concentrated choline. The obtained choline concentration of
0.75 297 μg/L was close to the SFC result and suggested that
extraction in online SFE was performed efficiently. For
0.50 acetylcholine, a calulation result of 1.7 μg/L was obtained
Choline from the peak area.
0.25 Acetylcholine
(×100,000)
0.00
5.0
0.0 2.5 5.0 7.5 10.0 12.5 min
Magnification
(×10,000) Acetylcholine
2.5
2.0 Choline
Acetylcholine
Choline 0.0
1.0
0 10 20 30 min
Online SFE-SFC Analysis of Choline and
0.0 Acetylcholine Standards
8.0 9.0 10.0 11.0 min (×100,000)
SFC Analysis of Choline and Acetylcholine
in a Cerebrospinal Fluid Sample
1.0
Online SFE-SFC-MS/MS Analysis Acetylcholine Choline
Next, a sample was prepared by impregnating cerebrospinal
fluid sample into filter paper and drying the paper. SFE-SFC-
MS/MS analysis was then performed on the sample. The
convenience of this method is gaining attention not only 0.0
because of easy of sample handling but also because of
improved miscibility concerns between a mobile phase of low
polar supercritical carbon dioxide and an aqueous sample 20.0 21.0 22.0 23.0 24.0 min
solvent containing a biological sample. Table 4 lists the
conditions used in online SFE-SFC-MS/MS analysis. Online SFE-SFC Analysis of Choline and
Acetylcholine in a Cerebrospinal Fluid Sample
First Edition: Mar. 2017
For Research Use Only. Not for use in diagnostic procedure.
This publication may contain references to products that are not available in your country. Please contact us to check the availability of these
products in your country.
The content of this publication shall not be reproduced, altered or sold for any commercial purpose without the written approval of Shimadzu.
Company names, product/service names and logos used in this publication are trademarks and trade names of Shimadzu Corporation or its
affiliates, whether or not they are used with trademark symbol “TM” or “£”. Third-party trademarks and trade names may be used in this
publication to refer to either the entities or their products/services. Shimadzu disclaims any proprietary interest in trademarks and trade names
www.shimadzu.com/an/ other than its own.
The information contained herein is provided to you "as is" without warranty of any kind including without limitation warranties as to its
accuracy or completeness. Shimadzu does not assume any responsibility or liability for any damage, whether direct or indirect, relating to the
use of this publication. This publication is based upon the information available to Shimadzu on or before the date of publication, and subject
to change without notice.
© Shimadzu Corporation, 2017