Page 23 - Pharmaceutical Solution for Pharma Analysis
P. 23
Application No. L520
News
Analysis of Montelukast Sodium Using Correction of System Volume Utilizing the
Nexera-i MT ACTO Function
Nexera-i MT has two flow channels of HPLC and UHPLC Here we introduce an example of method transfer from
and enables method switching from HPLC to UHPLC another LC system using the ACTO (Analytical Condition
within a single instrument. In this analysis, montelukast Transfer and Optimization) function, which is a standard
sodium was analyzed using the HPLC channel of Nexera-i feature of the Shimadzu integrated liquid chromatograph
MT. The obtained chromatogram is shown in Fig. 3 and "i-Series" and work station "LabSolutions".
indicates that the related substances listed in the JP are If an analytical method on an existing LC system is
also identified. Table 3 shows results of the system transferred to another LC system, the retention time may
suitability test.
not be identical due to the difference in dwell volume,
pump specifications, etc. In such a case, the gradient start
time adjustment function, which is a feature in the ACTO
mAU
150
function, can be executed to adjust the gradient start time
for the specified volume.
B
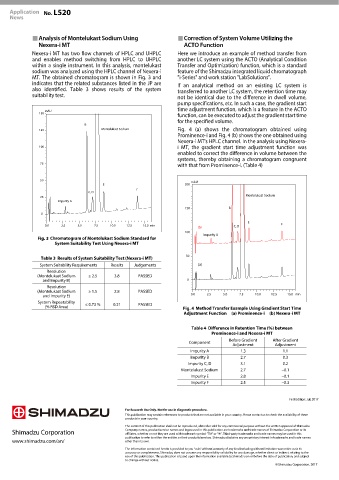
125 Montelukast Sodium Fig. 4 (a) shows the chromatogram obtained using
Prominence-i and Fig. 4 (b) shows the one obtained using
Nexera-i MT's HPLC channel. In the analysis using Nexera-
100 i MT, the gradient start time adjustment function was
enabled to correct the difference in volume between the
systems, thereby obtaining a chromatogram congruent
75
with that from Prominence-i. (Table 4)
50 mAU
E 200
F
C, D
Montelukast Sodium
25
Impurity A
150 B
0
E
0.0 2.5 5.0 7.5 10.0 12.5 15.0 min C, D F
(b)
100
Impurity A
Chromatogram of Montelukast Sodium Standard for
System Suitability Test Using Nexera-i MT
50
Table 3 Results of System Suitability Test (Nexera-i MT)
System Suitability Requirements Results Judgements (a)
Resolution
(Montelukast Sodium ≥ 2.5 3.8 PASSED
and Impurity B) 0
Resolution
(Montelukast Sodium ≥ 1.5 2.8 PASSED
and Impurity E) 0.0 2.5 5.0 7.5 10.0 12.5 15.0 min
System Repeatability ≤ 0.73 % 0.21 PASSED
(% RSD Area)
Method Transfer Example Using Gradient Start Time
Adjustment Function (a) Prominence-i (b) Nexera-i MT
Table 4 Difference in Retention Time (%) between
Prominence-i and Nexera-i MT
Before Gradient After Gradient
Component
Adjustment Adjustment
Impurity A 1.3 1.1
Impurity B 2.7 0.3
Impurity C, D 3.1 0.2
Montelukast Sodium 2.7 −0.1
Impurity E 2.8 −0.1
Impurity F 2.5 −0.3
First Edition: Jul. 2017
For Research Use Only. Not for use in diagnostic procedure.
This publication may contain references to products that are not available in your country. Please contact us to check the availability of these
products in your country.
The content of this publication shall not be reproduced, altered or sold for any commercial purpose without the written approval of Shimadzu.
Company names, product/service names and logos used in this publication are trademarks and trade names of Shimadzu Corporation or its
affiliates, whether or not they are used with trademark symbol “TM” or “”. Third-party trademarks and trade names may be used in this
publication to refer to either the entities or their products/services. Shimadzu disclaims any proprietary interest in trademarks and trade names
www.shimadzu.com/an/ other than its own.
The information contained herein is provided to you "as is" without warranty of any kind including without limitation warranties as to its
accuracy or completeness. Shimadzu does not assume any responsibility or liability for any damage, whether direct or indirect, relating to the
use of this publication. This publication is based upon the information available to Shimadzu on or before the date of publication, and subject
to change without notice.
© Shimadzu Corporation, 2017