Page 19 - Pharmaceutical Solution for Pharma Analysis
P. 19
Application No.L494
News
Table 4 Results of System Suitability Test Using USP Method (Original Method and Fast Method)
Analytical Conditions
System Suitability Requirements USP Original Method (Table 1) USP Fast Method (Table 3)
Results Judgments Results Judgments
USP Tailing Factor for Omeprazole ≦ 1.5 0.94 PASS 0.89 PASS
Rt 0.097 % PASS Rt 0.081 % PASS
Relative Standard Deviation for Omeprazole (n = 6) ≦ 1.0 %
Area 0.022 % PASS Area 0.121 % PASS
n Analysis According to Japanese Pharmacopeia
The analytical conditions specified in the 16th Edition of mV
the Japanese Pharmacopeia are shown in Table 5. For Omeprazole
the instrument, the integrated HPLC Prominence-i was
used. The system suitability test specified in the 4
Japanese Pharmacopeia includes three items, "Test for
required detectability", "System performance", and
"System repeatability". The respective chromatograms
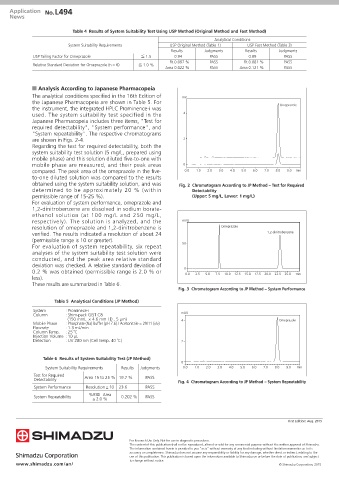
are shown in Figs. 2-4. 2
Regarding the test for required detectability, both the
system suitability test solution (5 mg/L, prepared using
mobile phase) and this solution diluted five-to-one with
mobile phase are measured, and their peak areas 0
compared. The peak area of the omeprazole in the five- 0.0 1.0 2.0 3.0 4.0 5.0 6.0 7.0 8.0 9.0 min
to-one diluted solution was compared to the results
obtained using the system suitability solution, and was Fig. 2 Chromatogram According to JP Method — Test for Required
determined to be approximately 20 % (within Detectability
permissible range of 15-25 %). (Upper: 5 mg/L, Lower: 1 mg/L)
For evaluation of system performance, omeprazole and
1,2-dinitrobenzene are dissolved in sodium borate-
ethanol solution (at 100 mg/L and 250 mg/L,
respectively). The solution is analyzed, and the mAU
resolution of omeprazole and 1,2-dinitrobenzene is Omeprazole
verified. The results indicated a resolution of about 24 1,2-dinitrobenzene
(permissible range is 10 or greater).
For evaluation of system repeatability, six repeat 50
analyses of the system suitability test solution were
conducted, and the peak area relative standard
deviation was checked. A relative standard deviation of 0
0.2 % was obtained (permissible range is 2.0 % or 0.0 2.5 5.0 7.5 10.0 12.5 15.0 17.5 20.0 22.5 25.0 min
less).
These results are summarized in Table 6.
Fig. 3 Chromatogram According to JP Method — System Performance
Table 5 Analytical Conditions (JP Method)
System : Prominece-i mAU
Column : Shim-pack GIST C8
(150 mmL. × 4.6 mm I.D., 5 µm) 4 Omeprazole
Mobile Phase : Phosphate (Na) Buffer (pH 7.6) / Acetonitrile = 29/11 (v/v)
Flowrate : 1.3 mL/min
Column Temp. : 25 ˚C
Injection Volume : 10 µL
Detection : UV 280 nm (Cell temp. 40 ˚C) 2
Table 6 Results of System Suitability Test (JP Method)
0
System Suitability Requirements Results Judgments 0.0 1.0 2.0 3.0 4.0 5.0 6.0 7.0 8.0 9.0 min
Test for Required Area 15 to 25 % 19.7 % PASS
Detectability
Fig. 4 Chromatogram According to JP Method — System Repeatability
System Performance Resolution ≧ 10 23.6 PASS
System Repeatability %RSD Area 0.202 % PASS
≦ 2.0 %
First Edition: Aug. 2015
For Research Use Only. Not for use in diagnostic procedures.
The content of this publication shall not be reproduced, altered or sold for any commercial purpose without the written approval of Shimadzu.
The information contained herein is provided to you "as is" without warranty of any kind including without limitation warranties as to its
accuracy or completeness. Shimadzu does not assume any responsibility or liability for any damage, whether direct or indirect, relating to the
use of this publication. This publication is based upon the information available to Shimadzu on or before the date of publication, and subject
to change without notice.
www.shimadzu.com/an/ © Shimadzu Corporation, 2015