Page 15 - Pharmaceutical Solution for Pharma Analysis
P. 15
Application No. C143
News
A calibration curve was created from the control blood and accuracy in the entire range, including the
plasma with standards added and the integrity of quantitative lower limit, was within 100±15 %. In the
accuracy and precision were evaluated. Good linearity same manner, precision (%RSD) was within 15 % and
was obtained in the set concentration range for all TKIs good repeatability was obtained (Table 2).
Table 2 Integrity Evaluation Results for Simultaneous Analysis of EGFR-TKIs, ALK-TKIs, and Metabolites
Range Accuracy (%) Precision (%RSD, n=5)
Compounds
(ng/mL) LLOQ Low *2 Medium *3 High *4 LLOQ *1 Low Medium High *4
*1
*2
*3
Afatinib 5-2000 98.5 103.2 106.3 95.7 12.4 13.9 7.6 7.4
Erlotinib 5-2000 95.1 103.2 102.7 92.3 4.7 5.4 0.8 2.0
OSI-420 5-2000 95.1 104.3 103.4 91.9 9.0 2.7 2.7 3.2
Gefitinib 5-2000 93.0 110.2 103.9 93.5 12.1 6.4 3.4 1.3
Osimertinib 5-2000 94.5 107.0 103.5 93.1 4.6 3.7 2.4 1.9
AZ5104 5-2000 94.7 106.5 102.7 92.5 9.8 6.0 2.7 3.1
Alectinib 5-2000 96.9 101.4 104.0 93.1 13.4 6.3 1.8 1.9
Crizotinib 5-2000 95.4 106.1 106.6 95.3 10.9 11.1 4.1 2.5
Ceritinib 10-2000 94.4 105.2 103.6 91.6 8.0 6.7 1.7 2.8
*1: 5 ng/mL (10 ng/mL for Ceritinib), *2: 10 ng/mL (25 ng/mL for Ceritinib), *3: 100 ng/mL, *4: 1000 ng/mL
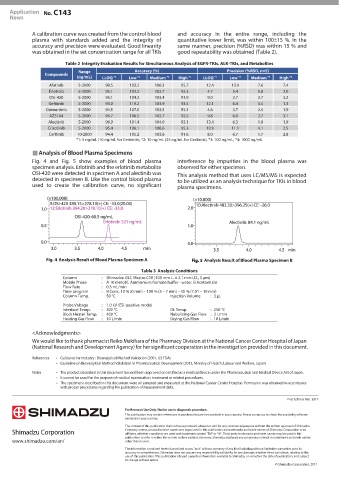
Analysis of Blood Plasma Specimens
Fig. 4 and Fig. 5 show examples of blood plasma interference by impurities in the blood plasma was
specimen analysis. Erlotinib and the erlotinib metabolite observed for either specimen.
OSI-420 were detected in specimen A and alectinib was This analysis method that uses LC/MS/MS is expected
detected in specimen B. Like the control blood plasma to be utilized as an analysis technique for TKIs in blood
used to create the calibration curve, no significant plasma specimens.
Analysis Result of Blood Plasma Specimen A Analysis Result of Blood Plasma Specimen B
Table 3 Analysis Conditions
Column : Shimadzu GLC Mastro C18 (100 mm L. × 2.1 mm I.D., 3 μm)
Mobile Phase : A 10 mmol/L Ammonium formate buffer - water, B Acetonitrile
Flow Rate : 0.3 mL/min
Time program : B Conc. 10 % (0 min) – 100 % (5 – 7 min) – 10 % (7.01 – 10 min)
Column Temp. 50 °C Injection Volume : 3 μL
Probe Voltage : 1.0 kV (ESI-positive mode)
Interface Temp. : 300 °C DL Temp. : 250 °C
Block Heater Temp. : 400 °C Nebulizing Gas Flow : 3 L/min
Heating Gas Flow : 10 L/min Drying Gas Flow : 10 L/min
<Acknowledgments>
We would like to thank pharmacist Reiko Makihara of the Pharmacy Division at the National Cancer Center Hospital of Japan
(National Research and Development Agency) for her significant cooperation in the investigation provided in this document.
References • Guidance for Industry : Bioanalytical Method Validation (2001, US FDA)
• Guideline on Bioanalytical Method Validation in Pharmaceutical Development (2013, Ministry of Health, Labour and Welfare, Japan)
Notes • The product described in this document has not been approved or certified as a medical device under the Pharmaceutical and Medical Device Act of Japan.
• It cannot be used for the purpose of medical examination, treatment or related procedures.
• The specimens described in this document were all sampled and measured at the National Cancer Center Hospital. Permission was obtained in accordance
with proper procedures regarding the publication of measurement data.
First Edition: Mar. 2017
For Research Use Only. Not for use in diagnostic procedure.
This publication may contain references to products that are not available in your country. Please contact us to check the availability of these
products in your country.
The content of this publication shall not be reproduced, altered or sold for any commercial purpose without the written approval of Shimadzu.
Company names, product/service names and logos used in this publication are trademarks and trade names of Shimadzu Corporation or its
affiliates, whether or not they are used with trademark symbol “TM” or “£”. Third-party trademarks and trade names may be used in this
publication to refer to either the entities or their products/services. Shimadzu disclaims any proprietary interest in trademarks and trade names
www.shimadzu.com/an/ other than its own.
The information contained herein is provided to you "as is" without warranty of any kind including without limitation warranties as to its
accuracy or completeness. Shimadzu does not assume any responsibility or liability for any damage, whether direct or indirect, relating to the
use of this publication. This publication is based upon the information available to Shimadzu on or before the date of publication, and subject
to change without notice.
© Shimadzu Corporation, 2017