Page 48 - LC-SFC_Pharma_Brochure
P. 48
Solution 5: Impurity Identification
In recent years, regulations have become increasingly strict with the requirements for impurities in pharmaceuticals.
For example, the International Council for Harmonization (ICH) Q3B (R2) (Impurities in New Drug Products) requires
identification and characterization of all degradation products, even at trace levels.
Various mass spectrometry with excellent qualitative capabilities are excellent tools for impurity identification. However,
the different operating requirements and compatibility issues of the chromatography and mass spectrometry systems have,
to some extent, proved to be challenging and have increased the workload.
Shimadzu offers a sophisticated 2D-LC system and uses it in conjunction with our MS to help you efficiently eliminate the
difficulties in impurity identification.
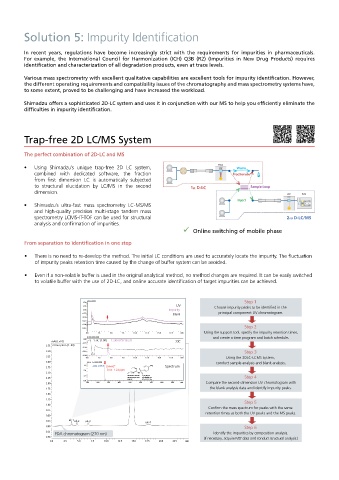
Trap-free 2D LC/MS System
The perfect combination of 2D-LC and MS
• Using Shimadzu’s unique trap-free 2D LC system, PDA Waste
combined with dedicated software, the fraction Fractionate
from first dimension LC is automatically subjected
to structural elucidation by LC/MS in the second 1st D-LC Sample Loop
dimension. UV MS
Inject
• Shimadzu’s ultra-fast mass spectrometry LC-MS/MS
and high-quality precision multi-stage tandem mass
spectrometry LCMS-IT-TOF can be used for structural 2nd D-LC/MS
analysis and confirmation of impurities.
Online switching of mobile phase
From separation to identification in one step
• There is no need to re-develop the method. The initial LC conditions are used to accurately locate the impurity. The fluctuation
of impurity peaks retention time caused by the change of buffer system can be avoided.
• Even if a non-volatile buffer is used in the original analytical method, no method changes are required. It can be easily switched
to volatile buffer with the use of 2D-LC, and online accurate identification of target impurities can be achieved.
uV (x1,000)
1.50 Step 1
1.25 UV Choose impurity peaks to be identified in the
Impurity
1.00
0.75 Blank principal component UV chromatogram.
0.50
0.25
0.00
-0.25 Step 2
0.0 2.5 5.0 7.5 10.0 12.5 15.0 17.5 min Using the support tool, specify the impurity retention times,
(x100,000,000) and create a time program and batch schedule.
1.00 1:TIC (1.00) 1:265.0757 (8.21)
mAU( x10) XIC
0.75
3.75 270nm,4nm (1. 00)
0.50
3.50 0.25 Step 3
3.25 0.00 0.0 2.5 5.0 7.5 10.0 12.5 15.0 17.5 min Using the 2DLC-LCMS system,
3.00 Inten. (x1,000,000) conduct sample analysis and blank analysis.
7.5 265.0757 [M+H] + NH 2
2.75 N Spectrum
5.0 Error: 1.24 ppm O S
2.50 3 C H N N H O
2.5 Molecular Formula = C 11H 12N 4O 2S
2.25 Monoisotopic Mass = 264.068096 Da Step 4
0.0 [M+H] + = 265.075372 Da
2.00 100 200 300 400 500 600 700 800 900 m/z Compare the second-dimension UV chromatogram with
1.75 the blank analysis data and identify impurity peaks.
1.50
1.25
Step 5
1.00
Confirm the mass spectrum for peaks with the same
0.75
retention times as both the UV peaks and the MS peaks.
0.50
0.25 uk-1 uk-2 uk-3 uk-4
0.00 Step 6
0.25 Identify the impurities by composition analysis.
PDA chromatogram (270 nm)
0.50 (If necessary, acquire MS data and conduct structural analysis.)
n
0.0 2.5 5.0 7.5 10.0 12.5 15.0 17.5 20.0 22.5 min