Page 13 - LC-SFC_Pharma_Brochure
P. 13
3. Results
3. Results
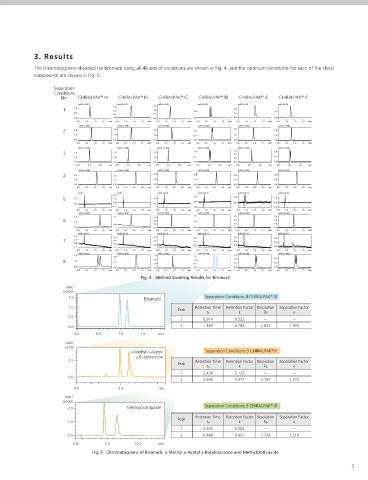
The chromatograms obtained for bromacil using all 48 sets of conditions are shown in Fig. 4, and the optimum conditions for each of the chiral
compounds are shown in Fig. 5.
Separation
Conditions
®
®
®
®
®
®
No. CHIRALPAK IA CHIRALPAK IB CHIRALPAK IC CHIRALPAK ID CHIRALPAK IE CHIRALPAK IF
mAU (×100) mAU (×10) 1.5 mAU (×100) mAU (×10) mAU (×10) mAU (×10)
1 1.0 7.5 1.0 5.0 5.0 5.0
5.0
0.5 2.5
2.5 0.5
0.0 0.0 0.0 0.0 0.0 0.0
0.0 1.0 2.0 3.0 min 0.0 1.0 2.0 3.0 min 0.0 1.0 2.0 3.0 min 0.0 1.0 2.0 3.0 min 0.0 1.0 2.0 3.0 min 0.0 1.0 2.0 3.0 min
mAU (×100) mAU (×100) mAU (×100) mAU (×100) mAU (×100) mAU (×100)
α α γ 2 2.0 2.0 2.0 1.0 2.0 2.0
1.0 1.0 1.0 0.5 1.0 1.0
0.0 0.0 0.0 0.0 0.0 0.0
0.0 0.5 1.0 1.5 min 0.0 0.5 1.0 1.5 min 0.0 0.5 1.0 1.5 min 0.0 0.5 1.0 1.5 min 0.0 0.5 1.0 1.5 min 0.0 0.5 1.0 1.5 min
mAU (×100) mAU (×100) mAU (×100) mAU (×100) mAU (×100) mAU (×100)
1.5
3 1.0 2.0 2.0 1.0 1.0 1.0
0.5 1.0 1.0 0.5 0.5 0.5
0.0 0.0 0.0 0.0 0.0 0.0
0.0 1.0 2.0 min 0.0 1.0 2.0 min 0.0 1.0 2.0 min 0.0 1.0 2.0 min 0.0 1.0 2.0 min 0.0 1.0 2.0 min
mAU (×100) mAU (×100) mAU (×100) mAU (×100) mAU (×100) mAU (×100)
3.0
4 2.0 2.0 2.0 2.0 2.0 2.0
1.0 1.0 1.0 1.0 1.0 1.0
0.0 0.0 0.0 0.0 0.0 0.0
0.0 1.0 2.0 3.0 min 0.0 1.0 2.0 3.0 min 0.0 1.0 2.0 3.0 min 0.0 1.0 2.0 3.0 min 0.0 1.0 2.0 3.0 min 0.0 1.0 2.0 3.0 min
mAU mAU mAU (×0.1) mAU (×0.1) mAU (×0.1) mAU (×0.1)
2.0 5.0
5 1.0 1.0 5.0 2.5 5.0
0.0 0.0 0.0 0.0
0.0 0.0
−1.0 −5.0
−5.0
0.0 1.0 2.0 3.0 min 0.0 1.0 2.0 3.0 min 0.0 1.0 2.0 3.0 min 0.0 1.0 2.0 3.0 min 0.0 1.0 2.0 3.0 min 0.0 1.0 2.0 3.0 min
mAU (×100) mAU (×100) mAU (×100) mAU (×100) mAU (×100) mAU (×100)
2.0
2.0 2.5 3.0 3.0 3.0
6 1.0 2.0 1.0 2.0 2.0
0.0 1.0 1.0 1.0
0.0 0.0 0.0 0.0 0.0
0.0 0.5 1.0 1.5 min 0.0 0.5 1.0 1.5 min 0.0 0.5 1.0 1.5 min 0.0 0.5 1.0 1.5 min 0.0 0.5 1.0 1.5 min 0.0 0.5 1.0 1.5 min
mAU (×0.1) mAU (×0.1) mAU (×0.1) mAU (×0.1) mAU (×0.1) mAU (×0.1)
5.0 5.0
5.0 5.0 5.0 5.0
7 2.5 2.5 2.5 2.5
0.0 2.5 2.5
0.0 0.0 0.0
−2.5 −2.5 0.0 0.0
0.0 1.0 2.0 3.0 min 0.0 1.0 2.0 3.0 min 0.0 1.0 2.0 3.0 min 0.0 1.0 2.0 3.0 min 0.0 1.0 2.0 3.0 min 0.0 1.0 2.0 3.0 min
mAU (×100) mAU (×100) mAU (×100) mAU (×100) mAU (×100) mAU (×100)
1.0 3.0 1.5 1.5
2.0 1.0
2.0 1.0 1.0
8 0.5 1.0
1.0 0.5 0.5 0.5
0.0 0.0 0.0 0.0 0.0 0.0
0.0 0.5 1.0 1.5 min 0.0 0.5 1.0 1.5 min 0.0 0.5 1.0 1.5 min 0.0 0.5 1.0 1.5 min 0.0 0.5 1.0 1.5 min 0.0 0.5 1.0 1.5 min
Fig. 4 Method Scouting Results for Bromacil
mAU
(×100)
®
1.5 Bromacil Separation Conditions 8 CHIRALPAK ID
1.0 Retention Time Retention Factor Resolution Separation Factor
Peak
tn k Rs α
0.5
1 0.974 0.522 - -
0.0 2 1.142 0.784 2.643 1.504
0.0 0.5 1.0 1.5 min
mAU
(×10)
®
α-Methyl-α-Acetyl- Separation Conditions 3 CHIRALPAK IF
γ-Butyloractone
2.5 Retention Time Retention Factor Resolution Separation Factor
Peak
tn k Rs α
1 2.434 3.125 - -
0.0
2 2.936 3.977 3.167 1.273
0.0 2.5 min
mAU
(×100)
®
Separation Conditions 3 CHIRALPAK ID
2.0 Methylclothiazide
Retention Time Retention Factor Resolution Separation Factor
Peak
1.0 tn k Rs α
1 4.465 6.202 - -
0.0 2 6.449 9.401 3.724 1.516
0.0 5.0 10.0 min
Fig. 5 Chromatograms of Bromacil, α-Methyl-α-Acetyl-γ-Butyloractone and Methylclothiazide
3