Page 10 - LC-SFC_Pharma_Brochure
P. 10
4-7. Determining Analytical Conditions for Simultaneous Analysis
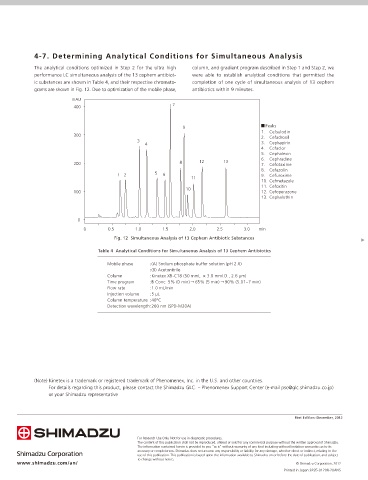
The analytical conditions optimized in Step 2 for the ultra high column, and gradient program described in Step 1 and Step 2, we
performance LC simultaneous analysis of the 13 cephem antibiot- were able to establish analytical conditions that permitted the
ic substances are shown in Table 4, and their respective chromato- completion of one cycle of simultaneous analysis of 13 cephem
grams are shown in Fig. 12. Due to optimization of the mobile phase, antibiotics within 9 minutes.
mAU
7
400
Peaks
9
1. Cefsulodin
300
2. Cefadroxil
3 3. Cephapirin
4
4. Cefaclor
5. Cephalexin
6. Cephradine
200 8 12 13 7. Cefotaxime
8. Cefazolin
12 5 6 9. Cefuroxime
11
10. Cefmetazole
11. Cefoxitin
10
100 12. Cefoperazone
13. Cephalothin
0
0 0.5 1.0 1.5 2.0 2.5 3.0 min
Fig. 12 Simultaneous Analysis of 13 Cephem Antibiotic Substances
Table 4 Analytical Conditions for Simultaneous Analysis of 13 Cephem Antibiotics
Mobile phase :(A) Sodium phosphate buffer solution (pH 2.6)
:(B) Acetonitrile
Column :Kinetex XB-C18 (50 mmL. × 3.0 mmI.D., 2.6 µm)
Time program :B Conc. 5% (0 min) 65% (5 min) 90% (5.01−7 min)
Flow rate :1.0 mL/min
Injection volume :5 µL
Column temperature :40°C
Detection wavelength :260 nm (SPD-M20A)
(Note) Kinetex is a trademark or registered trademark of Phenomenex, Inc. in the U.S. and other countries.
For details regarding this product, please contact the Shimadzu GLC. – Phenomenex Support Center (e-mail psc@glc.shimadzu.co.jp)
or your Shimadzu representative
First Edition: December, 2012
For Research Use Only. Not for use in diagnostic procedures.
The content of this publication shall not be reproduced, altered or sold for any commercial purpose without the written approval of Shimadzu.
The information contained herein is provided to you "as is" without warranty of any kind including without limitation warranties as to its
accuracy or completeness. Shimadzu does not assume any responsibility or liability for any damage, whether direct or indirect, relating to the
use of this publication. This publication is based upon the information available to Shimadzu on or before the date of publication, and subject
to change without notice.
www.shimadzu.com/an/ © Shimadzu Corporation, 2012
Printed in Japan 3295-01208-20ANS