Page 69 - Application Notebook - Solution for Food Safety
P. 69
Antimicrobial Screening System
i-Series Solution Package
Detects Synthetic Antimicrobial Agents of the Standard Residual
Concentration with High Sensitivity
Comprehensively Supports Processes from Pretreatment to Checking Results
This screening system consists of an i-Series LC system and a special kit that contains analytical methods and analytical columns in order to provide ready-to-use
screening of synthetic antimicrobial agents. It can reduce the time it takes for users to learn pretreatment procedures and examine conditions. Compound
names, retention times, UV spectra and other data of the regulated compounds are preregistered in the analytical methods for higher data reliability.
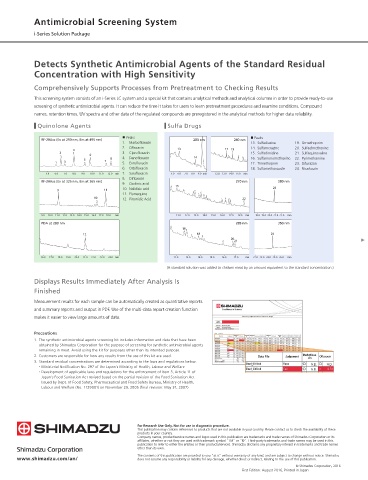
Quinolone Agents Sulfa Drugs
■ Peaks ■ Peaks
RF-20Axs (Ex at 290 nm, Em at 495 nm) 280 nm 240 nm
1. Marbofloxacin 13. Sulfadiazine 19. Ormethoprim
2. Ofloxacin 14. Sulfamerazine 20. Sulfadimethoxine
4 13 17 19
2 3. Ciprofloxacin 15. Sulfadimidine 21. Sulfaquinoxaline
6
5 7 8 4. Danofloxacin 14 16. Sulfamonomethoxine 22. Pyrimethamine
1 3 5. Enrofloxacin 17. Trimethoprim 23. Difurazon
6. Orbifloxacin 18. Sulfamethoxazole 24. Nicarbazin
5.0 6.0 7.0 8.0 9.0 10.0 11.0 12.0 min 7. Sarafloxacin 5.0 6.0 7.0 8.0 9.0 min 12.0 13.0 14.0 15.0 min
8. Difloxacin
RF-20Axs (Ex at 325 nm, Em at 365 nm) 270 nm 380 nm
9. Oxolinic acid 15
9 11 10. Nalidixic acid 23
11. Flumequine
10 12. Piromidic Acid 22
9.0 10.0 11.0 12.0 13.0 14.0 15.0 16.0 17.0 18.0 min 11.0 12.0 13.0 14.0 15.0 16.0 17.0 18.0 min 18.0 19.0 20.0 21.0 22.0 min
PDA at 280 nm 285 nm 350 nm
16
12 18 24
20
21
16.0 17.0 18.0 19.0 20.0 21.0 22.0 23.0 24.0 min 12.0 13.0 14.0 15.0 16.0 17.0 min 22.0 23.0 24.0 25.0 26.0 min
(A standard solution was added to chicken meat by an amount equivalent to the standard concentration.)
Displays Results Immediately After Analysis Is
Finished
Measurement results for each sample can be automatically created as quantitative reports
and summary reports and output in PDF. Use of the multi-data report creation function
makes it easier to view large amounts of data.
Precautions
1. The synthetic antimicrobial agents screening kit includes information and data that have been
obtained by Shimadzu Corporation for the purpose of screening for synthetic antimicrobial agents
remaining in meat. Avoid using the kit for purposes other than its intended purpose.
2. Customers are responsible for how any results from the use of this kit are used.
3. Standard residual concentrations are determined according to the laws and regulations below.
• Ministerial Notification No. 297 of the Japan's Ministry of Health, Labour and Welfare
• Development of applicable laws and regulations for the enforcement of Item 3, Article 11 of
Japan's Food Sanitation Act revised based on the partial revision of the Food Sanitation Act
Issued by Dept. of Food Safety, Pharmaceutical and Food Safety Bureau, Ministry of Health,
Labour and Welfare (No. 1129001) on November 29, 2005 (final revision: May 31, 2007)
For Research Use Only. Not for use in diagnostic procedure.
This publication may contain references to products that are not available in your country. Please contact us to check the availability of these
products in your country.
Company names, product/service names and logos used in this publication are trademarks and trade names of Shimadzu Corporation or its
affiliates, whether or not they are used with trademark symbol “TM” or “®”. Third-party trademarks and trade names may be used in this
publication to refer to either the entities or their products/services. Shimadzu disclaims any proprietary interest in trademarks and trade names
other than its own.
The contents of this publication are provided to you “as is” without warranty of any kind, and are subject to change without notice. Shimadzu
www.shimadzu.com/an/ does not assume any responsibility or liability for any damage, whether direct or indirect, relating to the use of this publication.
© Shimadzu Corporation, 2016
First Edition: August 2016, Printed in Japan