Page 54 - Application Notebook - Solution for Food Development
P. 54
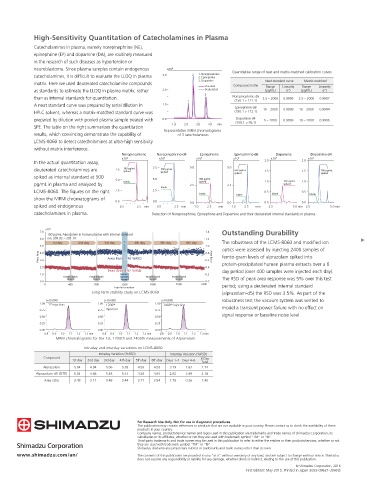
High-Sensitivity Quantitation of Catecholamines in Plasma
Catecholamines in plasma, namely norepinephrine (NE),
epinephrine (EP) and dopamine (DA), are routinely measured
in the research of such diseases as hypertension or
neuroblastoma. Since plasma samples contain endogenous ×10 6 Quantitative range of neat and matrix-matched calibration curves
catecholamines, it is difcult to evaluate the LLOQ in plasma 3.0 2 1. Norepinephrine
2. Epinephrine
matrix. Here we used deuterated catecholamine compounds 3. Dopamine Neat standard curve Matrix-matched
Standard Compound name Range Linearity Range Linearity
as standards to estimate the LLOQ in plasma matrix, rather 2.0 Deuterated (pg/mL) (r 2 ) (pg/mL) (r 2 )
3 Norepinephrine-d6
than as internal standards for quantitation. 2.5 – 2000 0.9999 2.5 – 2000 0.9997
1 (158.1 > 111.1)
A neat standard curve was prepared by serial dilution in 1.0 Epinephrine-d6
HPLC solvent, whereas a matrix-matched standard curve was (190.1 > 172.1) 10 – 2000 0.9999 10 – 2000 0.9994
Dopamine-d4
prepared by dilution with pooled plasma sample treated with 0.0 (158.1 > 95.1) 5 – 2000 0.9999 10 – 2000 0.9995
2.0
min
3.0
1.0
4.0
SPE. The table on the right summarizes the quantitation Representative MRM chromatograms
results, which convincing demonstrate the capability of of 3 catecholamines
LCMS-8060 to detect catecholamines at ultra-high sensitivity
without matrix interference.
Norepinephrine Norepinephrine-d6 Epinephrine Epinephrine-d6 Dopamine Dopamine-d4
×10 4 ×10 4 ×10 5 ×10 5 2.0 ×10 5 2.0 ×10 5
In the actual quantitation assay,
deuterated catecholamines are 7.5 300 pg/mL 7.5 500 pg/mL 5.0 5.0 500 pg/mL 1.5 1.5 500 pg/mL
spiked
spiked
spiked
spiked
spiked as internal standard at 500
5.0 blank 5.0 300 pg/mL 1.0 300 pg/mL 1.0
spiked
pg/mL in plasma and analyzed by 2.5 2.5 spiked
blank
LCMS-8060. The gures on the right 2.5 2.5 0.5 0.5
blank blank blank blank
show the MRM chromatograms of
0.0 0.0 0.0 0.0 0.0 0.0
spiked and endogenous 0.5 2.5 min 0.5 2.5 min 1.0 2.5 min 1.0 2.5 min 2.5 5.0 min 2.5 5.0min
catecholamines in plasma. Detection of Norepinephrine, Epinephrine and Dopamine and their deuterated internal standards in plasma.
×10 4
7.0 1.4 Outstanding Durability
100 pg/mL Alprazolam in human plasma with internal standard
6.0 m/z 309.05 > 281.10 1.2
1st day 2nd day 3rd day 4th day 5th day 6th day The robustness of the LCMS-8060 and modied ion
5.0 A week 1.0
optics were assessed by injecting 2400 samples of
Peak Area 3.0 Area Ratio 3.46 %RSD 0.6 Area Ratio femto-gram levels of alprazolam spiked into
4.0
0.8
Area Ratio 3.46 %RSD
protein-precipitated human plasma extracts over a 6
2.0 0.4
Peak Area 4.97 %RSD day period (over 400 samples were injected each day).
1.0 Vacuum 0.2
Pause in batch Pause in batch system Pause in batch Pause in batch
analysis analysis vented analysis analysis The RSD of peak area response was 5% over this test
0.0 0.0
0 400 800 1200 1600 2000 2400 period; using a deuterated internal standard
Injection number
Long term stability study on LCMS-8060 (alprazolam-d5) the RSD was 3.5%. As part of the
(×10,000) (×10,000) (×10,000) robustness test the vacuum system was vented to
1.00 1 st injection 1.00 1200 th 1.00 2400 th injection
0.75 0.75 injection 0.75 model a transient power failure with no effect on
signal response or baseline noise level.
0.50 0.50 0.50
0.25 0.25 0.25
0.00 0.00 0.00
0.8 0.9 1.0 1.1 1.2 1.3 min 0.8 0.9 1.0 1.1 1.2 1.3 min 0.8 0.9 1.0 1.1 1.2 1.3 min
MRM chromatograms for the 1st, 1200th and 2400th measurements of Alprazolam
Intraday and interday variations on LCMS-8060
Intraday Variation (%RSD) Interday Variation (%RSD)
Compound 6 Day
1st day 2nd day 3rd day 4th day 5th day 6th day Days 1–3 Days 4–6
Total
Alprazolam 5.04 4.94 5.06 5.38 4.55 4.83 3.19 1.63 2.74
Alprazolam-d5 (ISTD) 5.04 4.68 5.48 5.31 4.26 4.91 2.62 1.89 2.18
Area ratio 3.48 3.11 3.48 3.44 3.71 3.54 1.79 0.26 1.40
For Research Use Only. Not for use in diagnostic procedures.
This publication may contain references to products that are not available in your country. Please contact us to check the availability of these
products in your country.
Company names, products/service names and logos used in this publication are trademarks and trade names of Shimadzu Corporation, its
subsidiaries or its afliates, whether or not they are used with trademark symbol “TM” or “®”.
Third-party trademarks and trade names may be used in this publication to refer to either the entities or their products/services, whether or not
they are used with trademark symbol “TM” or “®”.
Shimadzu disclaims any proprietary interest in trademarks and trade names other than its own.
www.shimadzu.com/an/ The contents of this publication are provided to you “as is” without warranty of any kind, and are subject to change without notice. Shimadzu
does not assume any responsibility or liability for any damage, whether direct or indirect, relating to the use of this publication.
© Shimadzu Corporation, 2016
First Edition: May 2015, Printed in Japan 3655-09621-30ANS