Page 34 - Application Handbook - Liquid Chromatography
P. 34
Application No.L468
News
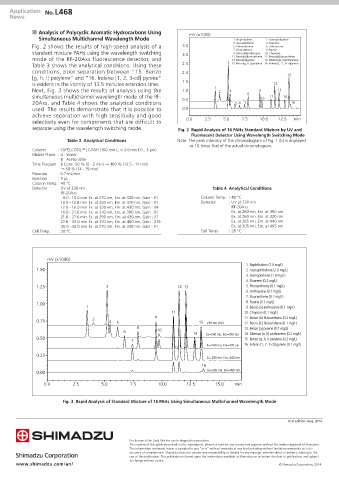
n Analysis of Polycyclic Aromatic Hydrocarbons Using mV (×1000)
Simultaneous Multichannel Wavelength Mode 1. Naphthalene 2. Acenaphthylene
3. Acenaphthene 4. Fluorene
Fig. 2 shows the results of high-speed analysis of a 3.5 5. Phenanthrene 6. Anthracene
8. Pyrene
standard mixture PAHs using the wavelength switching 3.0 7. Fluoranthene 10. Chrysene
9. Benzo[a]anthracene
mode of the RF-20Axs fluorescence detector, and 11. Benzo[b]fluoranthene 12. Benzo[k]fluoranthene
13. Benzo[a]pyrene
14. Dibenzo[a, h]anthracene
Table 3 shows the analytical conditions. Using these 2.5 3 15. Benzo[g, h, i]perylene 16. Indeno[1, 2, 3-cd]pyrene
conditions, poor separation between "15. Benzo 2.0
[g, h, i] perylene" and "16. Indeno [1, 2, 3-cd] pyrene" 15
is evident in the vicinity of 13.5 minutes retention time. 1.5 12
Next, Fig. 3 shows the results of analysis using the 1.0 1 2 4 9 13
simultaneous multichannel wavelength mode of the RF- 8 10 11 14
20Axs, and Table 4 shows the analytical conditions 0.5 5 6 7 16
used. The results demonstrate that it is possible to 0.0
achieve separation with high sensitivity and good
selectivity even for components that are difficult to 0.0 2.5 5.0 7.5 10.0 12.5 min
separate using the wavelength switching mode. Fig. 2 Rapid Analysis of 16 PAHs Standard Mixture by UV and
Fluorescent Detector Using Wavelength Switching Mode
Table 3 Analytical Conditions Note: The peak intensity of the chromatogram of Fig. 1 (b) is displayed
as 10 times that of the actual chromatogram.
Column : SUPELCOSIL LC-PAH (100 mm L. × 3.0 mm I.D., 3 μm)
TM
Mobile Phase : A : Water
B : Acetonitrile
Time Program : B Conc. 50 % (0 - 2 min) → 100 % (13.5 - 14 min)
→ 50 % (14 - 15 min)
Flowrate : 0.7 mL/min
Injection : 5 μL
Column Temp. : 40 °C
Detector : UV at 230 nm Table 4 Analytical Conditions
RF-20Axs
0.0 - 10.0 min Ex. at 270 nm, Em. at 330 nm, Gain : X1 Column Temp. : 40 °C
10.0 - 12.8 min Ex. at 250 nm, Em. at 370 nm, Gain : X1 Detector : UV at 230 nm
12.8 - 16.0 min Ex. at 330 nm, Em. at 430 nm, Gain : X4 RF-20Axs
16.0 - 21.0 min Ex. at 270 nm, Em. at 390 nm, Gain : X1 Ex. at 260 nm, Em. at 350 nm
21.0 - 27.6 min Ex. at 290 nm, Em. at 430 nm, Gain : X1 Ex. at 260 nm, Em. at 420 nm
27.6 - 30.0 min Ex. at 370 nm, Em. at 460 nm, Gain : X16 Ex. at 285 nm, Em. at 440 nm
30.0 - 40.0 min Ex. at 270 nm, Em. at 330 nm, Gain : X1 Ex. at 305 nm, Em. at 495 nm
Cell Temp. : 28 °C Cell Temp. : 28 °C
mV (×1000)
1. Naphthalene (1.0 mg/L)
1.50 2. Acenaphthylene (2.0 mg/L)
3. Acenaphthene (1.0 mg/L)
4. Fluorene (0.2 mg/L)
1.25 3 12 13 5. Phenanthrene (0.1 mg/L)
6. Anthracene (0.1 mg/L)
7. Fluoranthene (0.2 mg/L)
1.00 8. Pyrene (0.1 mg/L)
1 9. Benzo [a] anthracene (0.1 mg/L)
11 10. Chrysene (0.1 mg/L)
9 11. Benzo [b] fluoranthene (0.2 mg/L)
0.75 2 4 5 15 230 nm (UV) 12. Benzo [k] fluoranthene (0.1 mg/L)
8 13. Benzo [a] pyrene (0.1 mg/L)
6 10 14 Ex=260 nm, Em=350 nm 14. Dibenzo [a, h] anthracene (0.2 mg/L)
0.50 15. Benzo [g, h, i] perylene (0.2 mg/L)
7
Ex=260 nm, Em=420 nm 16. Indeno [1, 2, 3-cd] pyrene (0.1 mg/L)
0.25
Ex=285 nm, Em=440 nm
16
Ex=305 nm, Em=495 nm
0.00
0.0 2.5 5.0 7.5 10.0 12.5 15.0 min
Fig. 3 Rapid Analysis of Standard Mixture of 16 PAHs Using Simultaneous Multichannel Wavelength Mode
First Edition: Aug. 2014
For Research Use Only. Not for use in diagnostic procedures.
The content of this publication shall not be reproduced, altered or sold for any commercial purpose without the written approval of Shimadzu.
The information contained herein is provided to you "as is" without warranty of any kind including without limitation warranties as to its
accuracy or completeness. Shimadzu does not assume any responsibility or liability for any damage, whether direct or indirect, relating to the
use of this publication. This publication is based upon the information available to Shimadzu on or before the date of publication, and subject
to change without notice.
www.shimadzu.com/an/ © Shimadzu Corporation, 2014