Page 50 - Clinical-Medical Solution for Clinical Chemistry
P. 50
Integration of amino acid, acylcarnitine and steroids
analysis in single FIA/LC-MS/MS platform
The isotopically labeled internal standards for amino acids, acylcarnitines, and steroids were purchased from Cambridge
Isotope Laboratories, Inc. Quality control materials were obtained from the Newborn Screening Quality Assurance
Program at the Centers for Disease Control and Prevention (CDC).
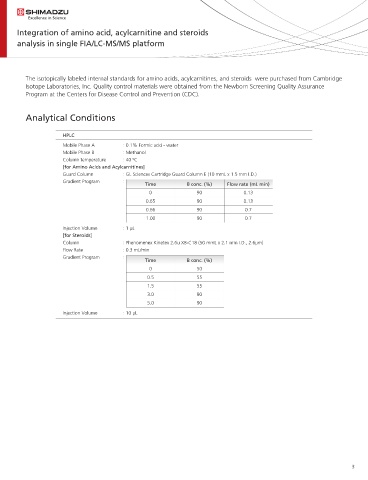
Analytical Conditions
HPLC
Mobile Phase A : 0.1% Formic acid - water
Mobile Phase B : Methanol
Column temperature : 40 ºC
[for Amino Acids and Acylcarnitines]
Guard Column : GL Sciences Cartridge Guard Column E (10 mmL x 1.5 mm I.D.)
Gradient Program :
Time B conc. (%) Flow rate (mL min)
0 90 0.13
0.65 90 0.13
0.66 90 0.7
1.00 90 0.7
Injection Volume : 1 µL
[for Steroids]
Column : Phenomenex Kinetex 2.6u XB-C18 (50 mmL x 2.1 mm I.D., 2.6µm)
Flow Rate : 0.3 mL/min
Gradient Program :
Time B conc. (%)
0 50
0.5 55
1.5 55
3.0 90
5.0 90
Injection Volume : 10 µL
3