Page 33 - Application Notebook - PFAS Analysis
P. 33
No. SSI-LCMS-106
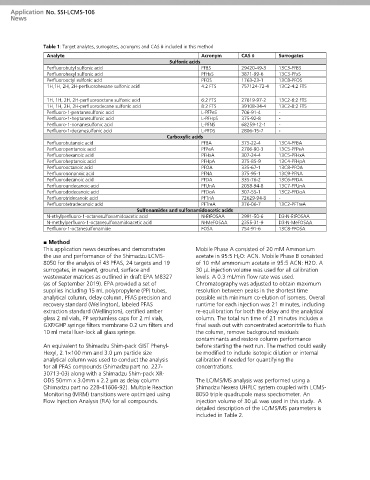
Table 1: Target analytes, surrogates, acronyms and CAS # included in this method
Analyte Acronym CAS # Surrogates
Sulfonic acids
Perfluorobutyl sulfonic acid PFBS 29420-49-3 13C3-PFBS
Perfluorohexyl sulfonic acid PFHxS 3871-99-6 13C3-PFxS
Perfluorooctyl sulfonic acid PFOS 1763-23-1 13C8-PFOS
1H,1H, 2H, 2H-perfluorohexane sulfonic acid 4:2 FTS 757124-72-4 13C2-4:2 FTS
1H, 1H, 2H, 2H-perfluorooctane sulfonic acid 6:2 FTS 27619-97-2 13C2-6:2 FTS
1H, 1H, 2H, 2H-perfluorodecane sulfonic acid 8:2 FTS 39108-34-4 13C2-8:2 FTS
Perfluoro-1-pentanesulfonic acid L-PFPeS 706-91-4 -
Perfluoro-1-heptanesulfonic acid L-PFHpS 375-92-8 -
Perfluoro-1-nonanesulfonic acid L-PFNS 68259-12-1 -
Perfluoro-1-decanesulfonic acid L-PFDS 2806-15-7 -
Carboxylic acids
Perfluorobutanoic acid PFBA 375-22-4 13C4-PFBA
Perfluoropentanoic acid PFPeA 2706-90-3 13C5-PFPeA
Perfluorohexanoic acid PFHxA 307-24-4 13C5-PFHxA
Perfluoroheptanoic acid PFHpA 375-85-9 13C4-PFHpA
Perfluorooctanoic acid PFOA 335-67-1 13C8-PFOA
Perfluorononanoic acid PFNA 375-95-1 13C9-PFNA
Perfluorodecanoic acid PFDA 335-76-2 13C6-PFDA
Perfluoroundecanoic acid PFUnA 2058-94-8 13C7-PFUnA
Perfluorododecanoic acid PFDoA 307-55-1 13C2-PFDoA
Perfluorotridecanoic acid PFTriA 72629-94-8 -
Perfluorotetradecanoic acid PFTreA 376-06-7 13C2-PFTreA
Sulfonamides and sulfonamidoacetic acids
N-ethylperfluoro-1-octanesulfonamidoacetic acid N-EtFOSAA 2991-50-6 D3-N-EtFOSAA
N-methylperfluoro-1-octanesulfonamidoacetic acid N-MeFOSAA 2355-31-9 D3-N-MeFOSAA
Perfluoro-1-octanesulfonamide FOSA 754-91-6 13C8-PFOSA
■ Method
This application news describes and demonstrates Mobile Phase A consisted of 20 mM Ammonium
the use and performance of the Shimadzu LCMS- acetate in 95:5 H2O: ACN. Mobile Phase B consisted
8050 for the analysis of 43 PFAS, 24 targets and 19 of 10 mM ammonium acetate in 95:5 ACN: H2O. A
surrogates, in reagent, ground, surface and 30 μL injection volume was used for all calibration
wastewater matrices as outlined in draft EPA M8327 levels. A 0.3 mL/min flow rate was used.
(as of September 2019). EPA provided a set of Chromatography was adjusted to obtain maximum
supplies including 15 mL polypropylene (PP) tubes, resolution between peaks in the shortest time
analytical column, delay column, PFAS precision and possible with minimum co-elution of isomers. Overall
recovery standard (Wellington), labeled PFAS runtime for each injection was 21 minutes, including
extraction standard (Wellington), certified amber re-equilibration for both the delay and the analytical
glass 2 ml vials, PP septumless caps for 2 ml vials, column. The total run time of 21 minutes includes a
GXF/GHP syringe filters membrane 0.2 um filters and final wash out with concentrated acetonitrile to flush
10 ml metal luer-lock all glass syringe. the column, remove background residuals
contaminants and restore column performance
An equivalent to Shimadzu Shim-pack GIST Phenyl- before starting the next run. The method could easily
Hexyl, 2.1×100 mm and 3.0 µm particle size be modified to include isotopic dilution or internal
analytical column was used to conduct the analysis calibration if needed for quantifying the
for all PFAS compounds (Shimadzu part no. 227- concentrations.
30713-03) along with a Shimadzu Shim-pack XR-
ODS 50mm x 3.0mm x 2.2 µm as delay column The LC/MS/MS analysis was performed using a
(Shimadzu part no 228-41606-92). Multiple Reaction Shimadzu Nexera UHPLC system coupled with LCMS-
Monitoring (MRM) transitions were optimized using 8050 triple quadrupole mass spectrometer. An
Flow Injection Analysis (FIA) for all compounds. injection volume of 30 µL was used in this study. A
detailed description of the LC/MS/MS parameters is
included in Table 2.