Page 34 - Application Notebook - PFAS Analysis
P. 34
No. SSI-LCMS-106
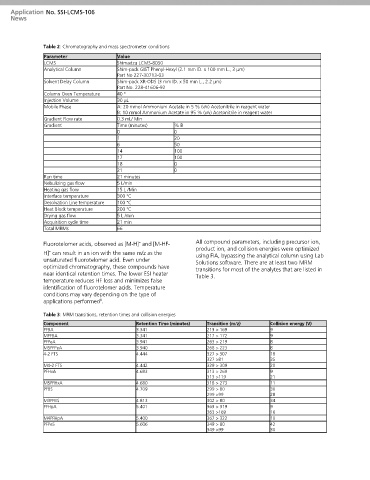
Table 2: Chromatography and mass spectrometer conditions
Parameter Value
LCMS Shimadzu LCMS-8050
Analytical Column Shim-pack GIST Phenyl-Hexyl (2.1 mm ID. x 100 mm L., 3 μm)
Part No 227-30713-03
Solvent Delay Column Shim-pack XR-ODS (3 mm ID. x 50 mm L., 2.2 μm)
Part No. 228-41606-92
Column Oven Temperature 40 º
Injection Volume 30 µL
Mobile Phase A: 20 mmol Ammonium Acetate in 5 % (v/v) Acetonitrile in reagent water
B: 10 mmol Ammonium Acetate in 95 % (v/v) Acetonitrile in reagent water
Gradient Flow rate 0.3 mL/ Min
Gradient Time (minutes) % B
0 0
1 20
6 50
14 100
17 100
18 0
21 0
Run time 21 minutes
Nebulizing gas flow 5 L/min
Heating gas flow 15 L /Min
Interface temperature 300 °C
Desolvation Line temperature 100 °C
Heat Block temperature 200 °C
Drying gas flow 5 L /min
Acquisition cycle time 21 min
Total MRMs 66
-
Fluorotelomer acids, observed as [M-H] and [M-HF- All compound parameters, including precursor ion,
-
H] can result in an ion with the same m/z as the product ion, and collision energies were optimized
using FIA, bypassing the analytical column using Lab
unsaturated fluorotelomer acid. Even under Solutions software. There are at least two MRM
optimized chromatography, these compounds have transitions for most of the analytes that are listed in
near identical retention times. The lower ESI heater Table 3.
temperature reduces HF loss and minimizes false
identification of fluorotelomer acids. Temperature
conditions may vary depending on the type of
9
applications performed .
Table 3: MRM transitions, retention times and collision energies
Component Retention Time (minutes) Transition (m/z) Collision energy (V)
PFBA 3.341 213 > 169 9
MPFBA 3.341 217 > 172 9
PFPeA 3.941 263 > 219 8
M5PFPeA 3.940 268 > 223 8
4-2 FTS 4.444 327 > 307 18
327 >81 35
M4-2 FTS 4.442 329 > 309 20
PFHxA 4.683 313 > 269 9
313 >119 21
M5PFHxA 4.680 318 > 273 11
PFBS 4.709 299 > 80 30
299 >99 28
M3PFBS 4.813 302 > 80 34
PFHpA 5.401 363 > 319 9
363 >169 16
M4PFHpA 5.400 367 > 322 10
PFPeS 5.606 349 > 80 42
349 >99 30