Page 12 - 2_Pharmaceutical industry
P. 12
SCA-130-205
This is why the TOC-V WP/WS is especially The software use is enabled via user access
suitable for TOC determination in the ultra- rights. It offers user accounts on four different
trace range. levels, each protected through own
passwords. The administrator can individually
■ Calibration: change access rights for each user. The
Method: NPOC software allows changing of login during
Acidification: 3% ongoing operation. This is especially
Sparge time: 3 minutes important for laboratories working under
Oxidizer: 1,5% multiple shift operation
Injection volume: 20,4 ml All software operations are stored
automatically in the audit trail. This happens
entirely in the background. Only when
existing parameters are changed is a user
comment required. Data storage takes place
in an MSDE database.
The TOC-Control V software simplifies the
implementation of the system suitability tests
using integrated templates for the creation of
calibration curves and the measurement of
the control sample.
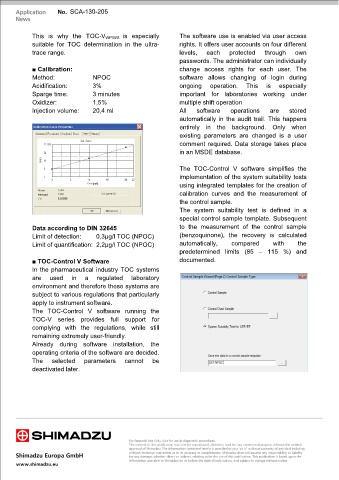
The system suitability test is defined in a
special control sample template. Subsequent
Data according to DIN 32645 to the measurement of the control sample
Limit of detection: 0,3µg/l TOC (NPOC) (benzoquinone), the recovery is calculated
Limit of quantification: 2,2µg/l TOC (NPOC) automatically, compared with the
predetermined limits (85 – 115 %) and
■ TOC-Control V Software documented.
In the pharmaceutical industry TOC systems
are used in a regulated laboratory
environment and therefore these systems are
subject to various regulations that particularly
apply to instrument software.
The TOC-Control V software running the
TOC-V series provides full support for
complying with the regulations, while still
remaining extremely user-friendly.
Already during software installation, the
operating criteria of the software are decided.
The selected parameters cannot be
deactivated later.