Page 8 - Pharmaceutical M5 Biopharma
P. 8
8 Sponsored Feature
Glycans Addressing both fast screening and full profiling
Glycosylation is one of the most common
post-translational modification of proteins.
The presence of the glycan moiety and its
structural profile must be monitored, since
it affects not only the effector function,
but also ADME and immunogenicity.
Quick Screening of Glycan Profile
Acquiring the glycan structure profile
at an early stage of biotherapeutic
development helps eliminate any
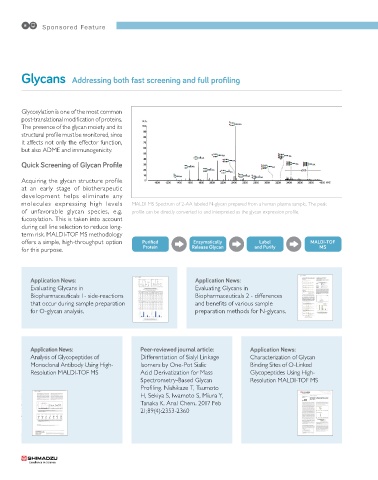
molecules expressing high levels MALDI MS Spectrum of 2-AA labeled N-glycan prepared from a human plasma sample. The peak
of unfavorable glycan species, e.g. profile can be directly converted to and interpreted as the glycan expression profile.
fucosylation. This is taken into account
during cell line selection to reduce long-
term risk. MALDI-TOF MS methodology
offers a simple, high-throughput option Purified Enzymatically Label MALDI-TOF
for this purpose. Protein Release Glycan and Purify MS
Application News: Application News:
Evaluating Glycans in Evaluating Glycans in
Biopharmaceuticals 1- side-reactions Biopharmaceuticals 2 - differences
that occur during sample preparation and benefits of various sample
for O-glycan analysis. preparation methods for N-glycans.
Application News: Peer-reviewed journal article: Application News:
Analysis of Glycopeptides of Differentiation of Sialyl Linkage Characterization of Glycan
Monoclonal Antibody Using High- Isomers by One-Pot Sialic Binding Sites of O-Linked
Resolution MALDI-TOF MS Acid Derivatization for Mass Glycopeptides Using High-
Spectrometry-Based Glycan Resolution MALDI-TOF MS
Profiling. Nishikaze T, Tsumoto
H, Sekiya S, Iwamoto S, Miura Y,
Tanaka K. Anal Chem. 2017 Feb
21;89(4):2353-2360