Page 7 - Pharmaceutical M5 Biopharma
P. 7
Sponsored Feature 7
Intact Proteins Robust and reliable separation and measurement
Charge Variant Analysis
Purified HPLC (IEX)
Protein
Modification, isomerization or cleavage
occurring on a charged amino acid
residue alters the overall charge of the
protein, affecting its structure, binding
affinity and stability. Hence charge-
related heterogeneity is an important
quality attribute of mAb.
The profile of charge variants
can be evaluated by ion exchange
chromatography (IEX), and this is
repeatedly monitored throughout the
developmental workflow.
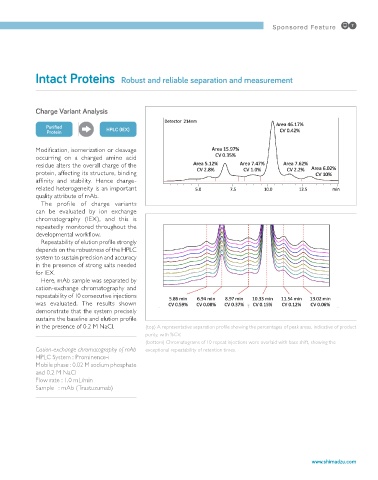
Repeatability of elution profile strongly
depends on the robustness of the HPLC
system to sustain precision and accuracy
in the presence of strong salts needed
for IEX.
Here, mAb sample was separated by
cation-exchange chromatography and
repeatability of 10 consecutive injections
was evaluated. The results shown
demonstrate that the system precisely
sustains the baseline and elution profile
in the presence of 0.2 M NaCl. (top) A representative separation profile showing the percentages of peak areas, indicative of product
purity, with %CV.
(bottom) Chromatograms of 10 repeat injections were overlaid with base shift, showing the
Cation-exchange chromatography of mAb exceptional repeatability of retention times.
HPLC System : Prominence-i
Mobile phase : 0.02 M sodium phosphate
and 0.2 M NaCl
Flow rate : 1.0 mL/min
Sample : mAb (Trastuzumab)
www.shimadzu.com