Page 2 - Pharmaceutical M5 Biopharma
P. 2
2 Sponsored Feature
Introduction
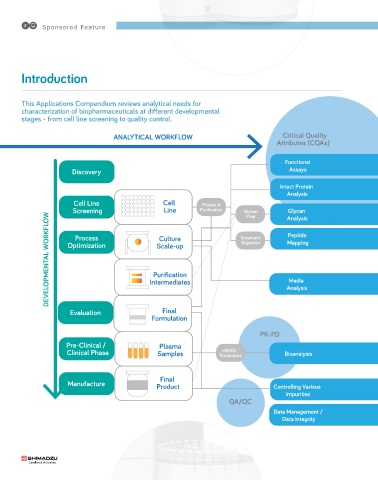
This Applications Compendium reviews analytical needs for
characterization of biopharmaceuticals at different developmental
stages - from cell line screening to quality control.
ANALYTICAL WORKFLOW Critical Quality
Attributes (CQAs)
Functional
Discovery Assays
Intact Protein Intact proteins: robust and reliable
Analysis separation and measurement
Cell Line Cell Protein A 4
Screenin Line Purification Glycan Analysis Glycans: addressin both fast screenin 7
Glycan
and full profilin
DEVELOPMENTAL WORKFLOW Optimization Intermediates Enzymatic Mappin Culture media: comprehensive analysis 10
Prep
Peptides: in-depth characterization of
Peptide
Process
Culture
primary structure
Di estion
9
Scale-up
Purification
Media
made fast and easy
Evaluation Final Analysis
Formulation
PK-PD
Pre-Clinical / Plasma Bioanalysis: acceleratin the
nSMOL™
Clinical Phase Samples Proteolysis Bioanalysis pre-clinical/clinical phase
13
Final Impurities: investi ation of a re ation
Manufacture Product Controllin Various and more 16
Impurities
QA/QC
Data Mana ement / Total solution for re ulatory compliance 18
and reportin
Data Inte rity
Global Shimadzu Location List 19