Page 17 - Clinical-Medical Solution for Clinical Chemistry
P. 17
Evaluation of Blood Lysis Procedures prior to Automated
Sample Preparation for Immunosuppressant Assay by LC-MS/MS
PLASMA WHOLE BLOOD PLASMA WHOLE BLOOD
Q 821.30>768.50 (+) 1.56e4 Q 821.30>768.50 (+) 1.73e5 ISTD 824.60>771.50 (+) 4.77e4 ISTD 824.60>771.50 (+) 4.89e4
TACROLIMUS 1.0e5 91% 1.0e5 13 C,D 2 -TACROLIMUS 4.0e4 4.0e4
2.0e4
2.0e4
0.0e0 0.0e0 0.0e0 0.0e0
0.50 0.75 1.00 0.50 0.75 1.00 0.50 0.75 1.00 0.50 0.75 1.00
Q 931.30>864.50 (+) 1.00e4 Q 931.30>864.50 (+) 7.30e4 ISTD 934.60>864.50 (+) 7.86e3 ISTD 934.60>864.50 (+) 7.66e3
86%
SIROLIMUS 5.0e4 5.0e4 D 3 -SIROLIMUS 5.0e3 5.0e3
0.0e0 0.0e0 0.0e0 0.0e0
0.50 0.75 1.00 0.50 0.75 1.00 0.50 0.75 1.00 0.50 0.75 1.00
Q 975.30>908.40 (+) 5.97e3 Q 975.30>908.40 (+) 7.18e4 ISTD 979.60>912.50 (+) 9.69e3 ISTD 979.60>912.50 (+) 9.36e3
EVEROLIMUS 5.0e4 92% 5.0e4 D 4 -EVEROLIMUS 5.0e3 5.0e3
2.5e4
2.5e4
0.0e0 0.0e0 0.0e0 0.0e0
0.50 0.75 1.00 0.50 0.75 1.00 0.50 0.75 1.00 0.50 0.75 1.00
PLASMA WHOLE BLOOD
Q 1219.70>1202.80 (+) 9.12e5 Q 1219.70>1202.80 (+) 2.19e6
2.0e6 59% 2.0e6
CYCLOSPORIN A 1.0e6 1.0e6
0.0e0 0.0e0
0.50 0.75 1.00 0.50 0.75 1.00
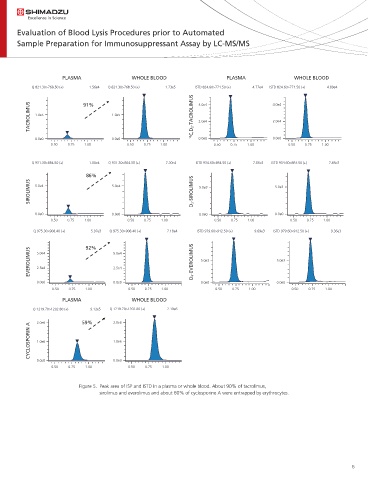
Figure 5. Peak area of ISP and ISTD in a plasma or whole blood. About 90% of tacrolimus,
sirolimus and everolimus and about 60% of cyclosporine A were entrapped by erythrocytes.
6