Page 15 - Clinical-Medical Solution for Clinical Chemistry
P. 15
Evaluation of Blood Lysis Procedures prior to Automated
Sample Preparation for Immunosuppressant Assay by LC-MS/MS
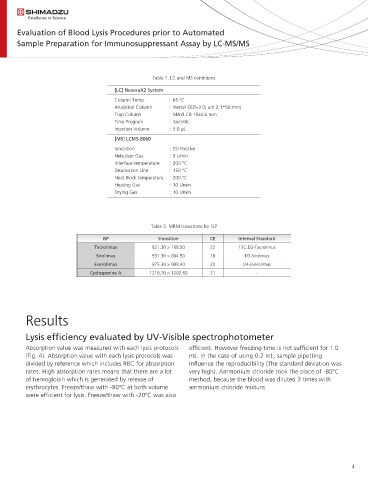
Table 1. LC and MS conditions
[LC] NexeraX2 System
Column Temp. : 65 °C
Analytical Column : Inertsil ODS-3 (5 um 2.1*50 mm)
Trap Column : MAYI C8 10x4.6 mm
Time Program : Isocratic
Injection Volume : 5.0 L
[MS] LCMS-8060
Ionization : ESI Positive
Nebulizer Gas : 3 L/min
Interface temperature : 200 °C
Desolvation Line : 150 °C
Heat Block temperature : 200 °C
Heating Gas : 10 L/min
Drying Gas : 10 L/min
Table 2. MRM transitions for ISP
ISP transition CE Internal Standard
Tacrolimus 821.30 > 768.50 22 13C,D2-Tacrolimus
Sirolimus 931.30 > 864.50 18 D3-Sirolimus
Everolimus 975.30 > 908.40 20 D4-Everolimus
Cyclosporine A 1219.70 > 1202.80 21 -
Results
Lysis ef ciency evaluated by UV-Visible spectrophotometer
Absorption value was measured with each lysis protocols ef cient. However freezing time is not suf cient for 1.0
(Fig. 4). Absorption value with each lysis protocols was mL. In the case of using 0.2 mL, sample pipetting
divided by reference which includes RBC for absorption in uence the reproducibility (The standard deviation was
rates. High absorption rates means that there are a lot very high). Ammonium chloride took the place of -80ºC
of hemoglobin which is generated by release of method, because the blood was diluted 3 times with
erythrocytes. Freeze/thaw with -80ºC at both volume ammonium chloride mixture.
were ef cient for lysis. Freeze/thaw with -20ºC was also
4