Page 13 - Shimadzu’s Solutions for Impurities Analysis
P. 13
FDA Regulations on
Genotoxic Impurities
Co-Sense for Impurities
Data
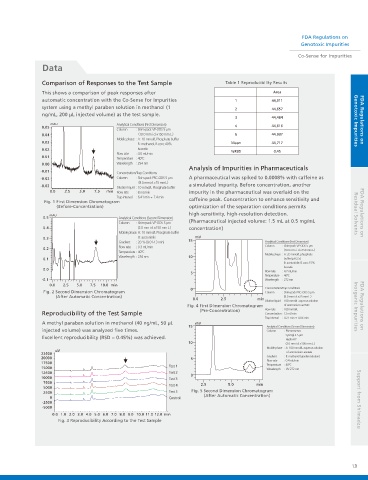
Comparison of Responses to the Test Sample Table 1 Reproducibility Results
This shows a comparison of peak responses after Area
automatic concentration with the Co-Sense for Impurities 1 44,911
system using a methyl paraben solution in methanol (1 2 44,657
ng/mL, 200 μL injected volume) as the test sample.
3 44,464 FDA Regulations on
mAU Analytical Conditions (First Dimension) Genotoxic Impurities
0.05 4 44,616
Column : Shim-pack VP-ODS 5 μm
0.04 (10.0 mm i.d.×150 mm L.) 5 44,937
Mobile phase : A: 10 mmol/L Phosphate buffer
0.03 Mean 44,717
B : methanol, B.conc 40%
0.02 Isocratic %RSD 0.45
Flow rate : 4.0 mL/min
0.01
Temperature : 40ºC
0.00 Wavelength : 254 nm
Analysis of Impurities in Pharmaceuticals
-0.01
Concentration/Trap Conditions
-0.02 Column : Shim-pack PRC-ODS 5 μm A pharmaceutical was spiked to 0.0008% with caffeine as
(8.0 mmi.d.×15 mmL.)
-0.03 a simulated impurity. Before concentration, another
Dilution liquid : 10 mmol/L Phosphate buffer
0.0 2.5 5.0 7.5 min Flow rate : 8 mL/min impurity in the pharmaceutical was overlaid on the
Trap interval : 6.41 min - 7.4 min
>
Fig. 1 First Dimension Chromatogram caffeine peak. Concentration to enhance sensitivity and
(Before-Concentration) optimization of the separation conditions permits
high-sensitivity, high-resolution detection. Residual Solvents FDA Regulations on
mAU
0.5 Analytical Conditions (Second Dimension)
Column : Shim-pack VP-ODS 5 μm (Pharmaceutical injected volume: 1.5 mL at 0.5 mg/mL
0.4 (2.0 mm i.d.×150 mm L.) concentration)
Mobile phase: A: 10 mmol/L Phosphate buffer
0.3 B: acetonitrile mV
Gradient : 20 % (8.01-13 min) 15 Analytical Conditions (First Dimension)
Flow rate : 0.3 mL/min Column : Shim-pack VP-ODS 5 μm
0.2 (10.0 mm i.d.×150 mm L.)
Temperature : 40ºC
Mobile phase : A: 20 mmol/L phosphate
Wavelength : 254 nm 10
0.1 buffer (pH 2.5)
B: acetonitrile, B.conc.15%
Isocratic
0.0 Flow rate : 4.7 mL/min
5
Temperature : 40ºC
-0.1 Wavelength : 272 nm
0.0 2.5 5.0 7.5 10.0 min
0 Concentration/Trap Conditions
Fig. 2 Second Dimension Chromatogram Column : Shim-pack PRC-ODS 5 μm
(After Automatic Concentration) (8.0 mm i.d.×15 mm L.)
0.0 2.5 min
Dilution liquid 100 mmol/L aqueous solution
Fig. 4 First Dimension Chromatogram of ammonium acetate Inorganic Impurities FDA Regulations on
(Pre-Concentration) Flow rate : 100 mmol/L
Reproducibility of the Test Sample Concentration : 12 mL/min
Trap interval : 4.21 min - 4.86 min
>
A methyl paraben solution in methanol (40 ng/mL, 50 μL mV
15 Analytical Conditions (Second Dimension)
injected volume) was analyzed five times. Column : Phenomenex
Synergi 2.5 μm
Excellent reproducibility (RSD = 0.45%) was achieved. Hydro-RP
10 (3.0 mm i.d.×100 mm L.)
Mobile phase : A: 100 mmol/L aqueous solution
uV
22500 of ammonium acetate
20000 5 Gradient : B: methanol (gradient elution)
17500 Flow rate : 0.4 mL/min
Test 1 Temperature : 40ºC
15000 Wavelength : UV 272 nm
12500 Test 2
0
10000 Test 3
7500 Test 4 2.5 5.0 min
5000
2500 Test 5 Fig. 5 Second Dimension Chromatogram
(After Automatic Concentration)
0 Control
-2500 Support from Shimadzu
-5000
0.0 1.0 2.0 3.0 4.0 5.0 6.0 7.0 8.0 9.0 10.0 11.0 12.0 min
Fig. 3 Reproducibility According to the Test Sample
13