Page 11 - Shimadzu’s Solutions for Impurities Analysis
P. 11
FDA Regulations on
Genotoxic Impurities
LCMS-2020
Data
Quantitative Analysis of Ultra-trace Impurities in Atropine
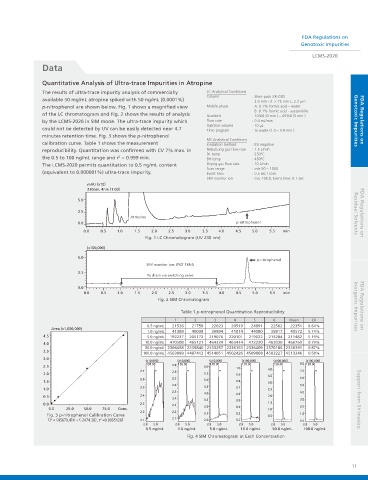
The results of ultra-trace impurity analysis of commercially LC Analytical Conditions
Column : Shim-pack XR-ODS
available 50 mg/mL atropine spiked with 50 ng/mL (0.0001%)
2.0 mm i.d. × 75 mm L, 2.2 μm
p-nitrophenol are shown below. Fig. 1 shows a magnified view Mobile phase : A: 0.1% formic acid – water
B: 0.1% formic acid - acetonitrile
of the LC chromatogram and Fig. 2 shows the results of analysis Gradient : 10%B (0 min ) – 45%B (5 min )
by the LCMS-2020 in SIM mode. The ultra-trace impurity which Flow rate : 0.4 mL/min Genotoxic Impurities FDA Regulations on
Injection volume : 10 μL
could not be detected by UV can be easily detected near 4.7 Time program : to waste (1.0 – 3.8 min )
minutes retention time. Fig. 3 shows the p-nitrophenol
MS Analytical Conditions
calibration curve. Table 1 shows the measurement Ionization method : ESI negative
reproducibility. Quantitation was confirmed with CV 7% max. in Nebulizing gas flow rate : 1.5 L/min
DL temp : 250ºC
the 0.5 to 100 ng/mL range and r = 0.999 min. BH temp : 450ºC
2
The LCMS-2020 permits quantitation to 0.5 ng/mL content Drying gas flow rate : 10 L/min
Scan range : m/z 50 – 1000
(equivalent to 0.000001%) ultra-trace impurity. Event time : 0.5 sec / scan
SIM monitor ion : m/z 138.0, Event time: 0.1 sec
mAU (x10)
230nm, 4nm (1.00)
5.0
2.5 Residual Solvents FDA Regulations on
Atropine
0.0 p-nitrophenol
0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0 4.5 5.0 5.5 min
Fig. 1 LC Chromatogram (UV 230 nm)
(x100,000)
5.0
p-nitrophenol
SIM monitor ion: m/z 138.0
2.5
To drain via switching valve
0.0
0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0 4.5 5.0 5.5 min
Fig. 2 SIM Chromatogram
Table 1 p-nitrophenol Quantitation Reproducibility Inorganic Impurities FDA Regulations on
1 2 3 4 5 6 Mean CV
0.5 ng/mL 21536 21759 22023 20519 24891 22562 22351 6.64%
Area (x1,000,000)
1.0 ng/mL 41389 40039 39994 41014 44080 36917 40572 5.74%
4.5 5.0 ng/mL 192237 205172 219076 220101 219022 213284 211482 5.19%
10.0 ng/mL 470508 465121 464324 465414 472220 463030 466769 0.79%
4.0
50.0 ng/mL 2306458 2335840 2333257 2336193 2336409 2370186 2336391 0.87%
3.5
100.0 ng/mL 4563089 4487412 4514051 4502426 4509868 4503227 4513346 0.58%
3.0
(x10,000) (x10,000) (x10,000) (x100,000) (x100,000) (x100,000)
138.00 138.00 138.00 138.00 138.00 8.0 138.00
2.5 2.9 6.0
1.0 4.0
2.7 2.8 7.0
2.0 5.5 0.9 3.5
2.6 2.7 5.0 6.0
1.5 0.8 3.0
2.6 5.0
2.5 4.5 0.7
1.0 2.5
2.5 4.0 0.6 4.0
0.5 2.4 2.0
2.4 3.0
3.5 0.5 Support from Shimadzu
0.0 2.3 2.3 1.5
0.0 25.0 50.0 75.0 Conc. 3.0 0.4 1.0 2.0
2.2 2.2 2.5 0.3
Fig. 3 p-nitrophenol Calibration Curve 0.5 1.0
(Y = (45679.4)X + (-2474.38), r =0.9995126) 2.1 2.1 2.0 0.2 0.0
2
2.8 5.0 2.8 5.0 2.8 5.0 2.8 5.0 2.8 5.0 2.8 5.0
0.5 ng/mL 1.0 ng/mL 5.0 ng/mL 10.0 ng/mL 50.0 ng/mL 100.0 ng/mL
Fig. 4 SIM Chromatogram at Each Concentration
11