Page 7 - Shimadzu’s Solutions for Impurities Analysis
P. 7
FDA Regulations on
Genotoxic Impurities
LCMS-IT-TOF ®
Data
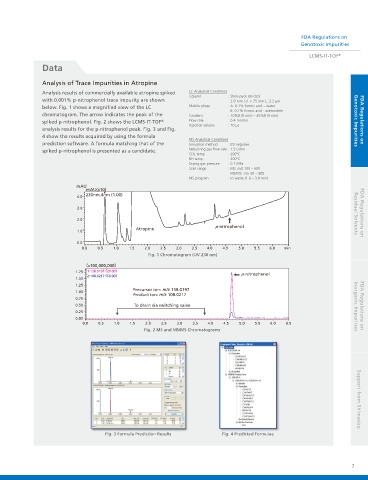
Analysis of Trace Impurities in Atropine
Analysis results of commercially available atropine spiked LC Analytical Conditions
Column : Shim-pack XR-ODS
with 0.001% p-nitrophenol trace impurity are shown 2.0 mm i.d. × 75 mm L, 2.2 μm
below. Fig. 1 shows a magnified view of the LC Mobile phase : A: 0.1% formic acid – water
B: 0.1% formic acid - acetonitrile
chromatogram. The arrow indicates the peak of the Gradient : 10%B (0 min) – 45%B (5 min)
spiked p-nitrophenol. Fig. 2 shows the LCMS-IT-TOF Flow rate : 0.4 mL/min Genotoxic Impurities FDA Regulations on
®
Injection volume : 10 μL
analysis results for the p-nitrophenol peak. Fig. 3 and Fig.
4 show the results acquired by using the formula
MS Analytical Conditions
prediction software. A formula matching that of the Ionization method : ESI negative
spiked p-nitrophenol is presented as a candidate. Nebulizing gas flow rate : 1.5 L/min
CDL temp : 200ºC
BH temp : 200ºC
Drying gas pressure : 0.1 MPa
Scan range : MS: m/z 100 – 600
MS/MS: m/z 50 – 500
MS program : to waste (1.0 – 3.8 min)
mAU
mAU(x10)
230nm,4nm (1.00)
230nm,4nm (1.00)
230nm,4nm (1.00)
4.0
3.0 Residual Solvents FDA Regulations on
2.0
p -nitrophenol
Atropine
1.0
0.0
0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0 4.5 5.0 5.5 6.0 min
Fig. 1 Chromatogram (UV 230 nm)
(x100,000,000)
1.75 1:138.0197 (20.00) p -nitrophenol
2:108.0217 (50.00)
1.50
1.25
Precursor ion: m/z 138.0197
1.00
Product ion: m/z 108.0217
0.75
0.50 To drain via switching valve Inorganic Impurities FDA Regulations on
0.25
0.00
0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0 4.5 5.0 5.5 6.0 6.5
Fig. 2 MS and MS/MS Chromatograms
Fig. 3 Formula Prediction Results Fig. 4 Predicted Formulae Support from Shimadzu
7