Page 3 - Shimadzu’s Solutions for Impurities Analysis
P. 3
Support for FDA Regulations on Genotoxic
Impurities in Pharmaceuticals
Genotoxicity is the property of a compound that can have At the end of 2008, the FDA issued a draft guidance,
irreversible effects on the structure and functionality of the "Genotoxic and Carcinogenic Impurities in Drug Substances
DNA in cells and cause DNA loss, DNA replication errors, and Products: Recommended Approaches." These
mutations, and chromosomal abnormalities. Addressing the guidelines establish and apply the Threshold of
issue of genotoxicity is one of the most important Toxicological Concern (TTC) to genotoxic impurities in the
safety-related issues during the development of new pharmaceutical ingredients. The TTC indicates the
pharmaceuticals. acceptable daily intake that presents no genetic risk to
The International Conference on Harmonization (ICH) humans below this threshold. It is effectively an estimated
guidelines indicated threshold values for general safety value such that the risk of developing cancer
impurities. However, the guidelines do not prescribe throughout the person’s life does not exceed one in a
numeric values for the genotoxicity of impurities, but million. It defines the acceptable daily intake as 1.5 μg
rather note comments such as "However, analytical max., assuming that the person takes the drug over 12
procedures should be developed for those potential months. (Table 1)
impurities that are expected to be unusually potent,
producing toxic or pharmacological effects at a level not For example, for a daily dose of 30 mg, the acceptable
more than (≤) the identification threshold." or "Lower daily intake concentration of the impurity is given by:
thresholds can be appropriate if the impurity is unusually Concentration Limit (ppm) = TTC (μg/day)/Dose (g/day) =
toxic." 1.5 (μg/day)/0.03 (g/day) = 50 x 10-6 (= 50 ppm).
Compared to the impurity value in the previous ICH
guidelines, this demands higher detection sensitivity for
the analysis of ultra-trace impurities.
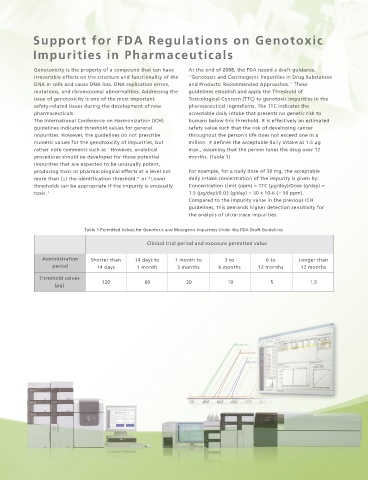
Table 1 Permitted Values for Genotoxic and Mutagenic Impurities Under the FDA Draft Guidelines
Clinical trial period and exposure permitted value
Administration Shorter than 14 days to 1 month to 3 to 6 to Longer than
period 14 days 1 month 3 months 6 months 12 months 12 months
Threshold values
120 60 20 10 5 1.5
(μg)