Page 23 - Shimadzu Journal vol.9 Issue2
P. 23
Biopharmaceutical
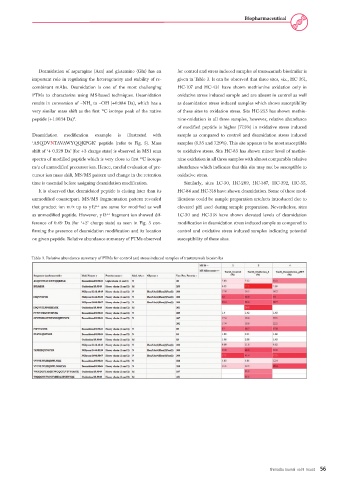
Deamidation of asparagine (Asn) and glutamine (Gln) has an for control and stress induced samples of trastuzumab biosimilar is
important role in regulating the heterogeneity and stability of re- given in Table 3. It can be observed that three sites, viz., HC-361,
combinant mAbs. Deamidation is one of the most challenging HC-107 and HC-431 have shown methionine oxidation only in
PTMs to characterize using MS-based techniques. Deamidation oxidative stress induced sample and are absent in control as well
results in conversion of –NH to –OH (+0.984 Da), which has a as deamidation stress induced samples which shows susceptibility
2
13
very similar mass shift as the first C isotope peak of the native of these sites to oxidation stress. Site HC-255 has shown methio-
peptide (+1.0034 Da) . 5 nine-oxidation in all three samples, however, relative abundance
of modified peptide is higher (77.5%) in oxidative stress induced
Deamidation modification example is illustrated with sample as compared to control and deamidation stress induced
‘ASQDVNTAVAWYQQKPGK’ peptide (refer to Fig. 5). Mass samples (6.35 and 7.29%). This site appears to be most susceptible
shift of ‘+ 0.329 Da’ (for +3 charge state) is observed in MS1 scan to oxidative stress. Site HC-83 has shown minor level of methio-
13
spectra of modified peptide which is very close to first C isotope nine oxidation in all three samples with almost comparable relative
m/z of unmodified precursor ion. Hence, careful evaluation of pre- abundance which indicates that this site may not be susceptible to
cursor ion mass shift, MS/MS pattern and change in the retention oxidative stress.
time is essential before assigning deamidation modification. Similarly, sites LC-30, HC-289, HC-387, HC-392, HC-55,
It is observed that deamidated peptide is eluting later than its HC-84 and HC-318 have shown deamidation. Some of these mod-
unmodified counterpart. MS/MS fragmentation pattern revealed ifications could be sample preparation artefacts introduced due to
++
that product ion m/z up to y12 are same for modified as well elevated pH used during sample preparation. Nevertheless, sites
++
as unmodified peptide. However, y13 fragment ion showed dif- LC-30 and HC-318 have shown elevated levels of deamidation
ference of 0.49 Da (for ‘+2’ charge state) as seen in Fig. 5 con- modification in deamidation stress induced sample as compared to
firming the presence of deamidation modification and its location control and oxidative stress induced samples indicating potential
on given peptide. Relative abundance summary of PTMs observed susceptibility of these sites.
Table 3. Relative abundance summary of PTMs for control and stress induced samples of trastuzumab biosimilar
56
Shimadzu Journal vol.9 Issue2 56