Page 21 - Shimadzu Journal vol.9 Issue2
P. 21
Biopharmaceutical
Table 2. Representative results of mass accuracies for peptide sequences of different chain length for control and stress induced samples of Trastuzumab biosimilar
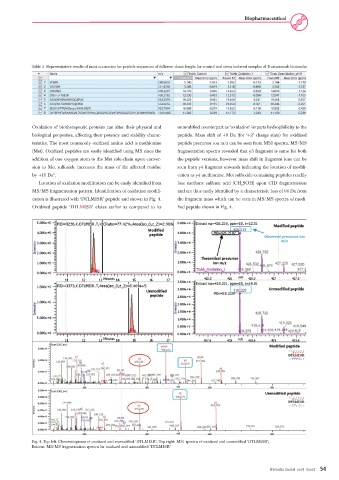
Oxidation of biotherapeutic proteins can alter their physical and unmodified counterpart as ‘oxidation’ imparts hydrophilicity to the
biological properties, affecting their potency and stability charac- peptide. Mass shift of +8 Da (for ‘+2’ charge state) for oxidized
teristics. The most commonly oxidized amino acid is methionine peptide precursor ion m/z can be seen from MS1 spectra. MS/MS
(Met). Oxidized peptides are easily identified using MS since the fragmentation spectra revealed that y3 fragment is same for both
addition of one oxygen atom to the Met side-chain upon conver- the peptide versions, however mass shift in fragment ions can be
sion to Met sulfoxide increases the mass of the affected residue seen from y4 fragment onwards indicating the location of modifi-
5
by +16 Da . cation as y4 methionine. Met sulfoxide-containing peptides readily
Location of oxidation modification can be easily identified from lose methane sulfenic acid (CH SOH) upon CID fragmentation
3
MS/MS fragmentation pattern. Identification of oxidation modifi- and are thus easily identified by a characteristic loss of 64 Da from
cation is illustrated with ‘DTLMISR’ peptide and shown in Fig. 4. the fragment mass which can be seen in MS/MS spectra of modi-
Oxidized peptide ‘DTLMISR’ elutes earlier as compared to its fied peptide shown in Fig. 4.
Fig. 4. Top left: Chromatograms of oxidized and unmodified ‘DTLMISR’; Top right: MS1 spectra of oxidized and unmodified ‘DTLMISR’;
Bottom: MS/MS fragmentation spectra for oxidized and unmodified ‘DTLMISR’
54
Shimadzu Journal vol.9 Issue2 54