Page 17 - Shimadzu Journal vol.9 Issue2
P. 17
Biopharmaceutical
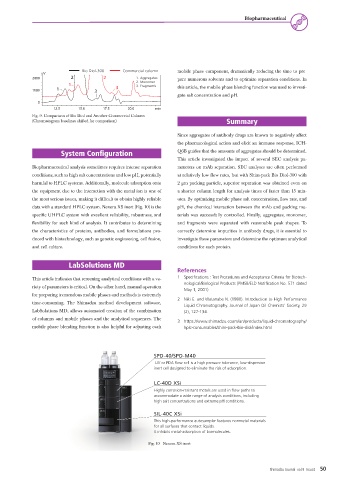
Bio Diol-300 Commercial column mobile phase component, dramatically reducing the time to pre-
uV
2000 2 2 1. Aggregates pare numerous solvents and to optimize separation conditions. In
2. Monomer
1 3. Fragments this article, the mobile phase blending function was used to investi-
1000 1 3 3
gate salt concentration and pH.
0
12.5 15.0 17.5 20.0 min
Fig. 9. Comparison of Bio Diol and Another Commercial Column
(Chromatogram baselines shifted for comparison) Summary
Since aggregates of antibody drugs are known to negatively affect
the pharmacological action and elicit an immune response, ICH-
System Configuration Q6B guides that the amounts of aggregates should be determined.
This article investigated the impact of several SEC analysis pa-
Biopharmaceutical analysis sometimes requires intense separation rameters on mAb separation. SEC analyses are often performed
conditions, such as high salt concentrations and low pH, potentially at relatively low flow rates, but with Shim-pack Bio Diol-300 with
harmful to HPLC systems. Additionally, molecule adsorption onto 2 µm packing particle, superior separation was obtained even on
the equipment due to the interaction with the metal ion is one of a shorter column length for analysis times of faster than 15 min-
the most serious issues, making it difficult to obtain highly reliable utes. By optimizing mobile phase salt concentration, flow rate, and
data with a standard HPLC system. Nexera XS inert (Fig. 10) is the pH, the chemical interaction between the mAb and packing ma-
specific UHPLC system with excellent reliability, robustness, and terials was successfully controlled. Finally, aggregates, monomer,
flexibility for such kind of analysis. It contributes to determining and fragments were separated with reasonable peak shapes. To
the characteristics of proteins, antibodies, and formulations pro- correctly determine impurities in antibody drugs, it is essential to
duced with biotechnology, such as genetic engineering, cell fusion, investigate these parameters and determine the optimum analytical
and cell culture. conditions for each protein.
LabSolutions MD
References
This article indicates that screening analytical conditions with a va- 1 Specifications : Test Procedures and Acceptance Criteria for Biotech
nological/Biological Products (PMSB/ELD Notification No. 571 dated
riety of parameters is critical. On the other hand, manual operation
May 1, 2001)
for preparing tremendous mobile phases and methods is extremely
2 Niki E. and Watanabe N. (1980). Introduction to High Performance
time-consuming. The Shimadzu method development software,
Liquid Chromatography. Journal of Japan Oil Chemists’ Society, 29
LabSolutions MD, allows automated creation of the combination (2), 127134.
of columns and mobile phases and the analytical sequences. The
3 https://www.shimadzu.coam/an/products/liquidchromatography/
mobile phase blending function is also helpful for adjusting each hplcconsumables/shimpackbiodiol/index.html
SPD-40/SPD-M40
UV or PDA flow cell is a high pressure tolerance, low-dispersion
inert cell designed to eliminate the risk of adsorption.
LC-40D XSi
Highly corrosion-resistant metals are used in flow paths to
accommodate a wide range of analysis conditions, including
high salt concentrations and extreme pH conditions.
SIL-40C XSi
This high-performance autosampler features nonmetal materials
for all surfaces that contact liquids.
It inhibits metal-adsorption of biomolecules.
Fig. 10 Nexera XS inert
Shimadzu Journal vol.9 Issue2 50