Page 7 - Shimadzu Co-Sense Series
P. 7
Analyzing Impurities in Antibody-Drug Conjugates Using Co-Sense for BA
Analyzing drug-derived impurities in pharmaceuticals containing antibody-drug conjugates (ADC) as a primary component requires
removing the antibodies (deproteinization) in order to achieve high-sensitivity analysis of low molecular impurities. Co-Sense for BA
used in combination with the Shimadzu Shim-pack MAYI series columns is able to efficiently analyze the impurities in antibody-drug
conjugates, for example, by injecting large volumes of samples that are deproteinized on-line.
Issues with Analyzing Impurities in Antibody-Drug Conjugates
To prevent cross contamination, impurity analysis should ideally injections. In addition, for samples with a high organic solvent
involve as simple a process as possible and with high-sensitivity ratio after deproteinization, the recovery rate may decrease
detection. However, when analyzing impurities in with the traditional trap injection method due to the impact of
antibody-drug conjugates, deproteinization is required to the sample solvent. Furthermore, if on-line pretreatment
eliminate high-concentration antibodies. If samples are (on-line solid phase extraction) is performed in order to
pretreated by deproteinization using organic solvents or acids, automate deproteinization, the recovery rate of target
the impact of the sample solvent can often worsen peak shapes, impurities may decrease due to interactions between antibodies
making it difficult to increase sensitivity through large-volume and drug-derived impurities.
On-line Deproteinization Using the Shim-pack MAYI-ODS Column
With the Co-Sense for BA system, components can be trapped reliably to enhance sensitivity, even with large-volume injections, due
to antibody removal (deproteinization) and the suppression of antibody and impurity interactions. The following shows an example
of analyzing approximately 0.07 % (molar ratio) of impurities present in a 1 mg/mL antibody solution. By eliminating the impacts of
antibodies, a recovery rate of approximately 100 % was achieved.
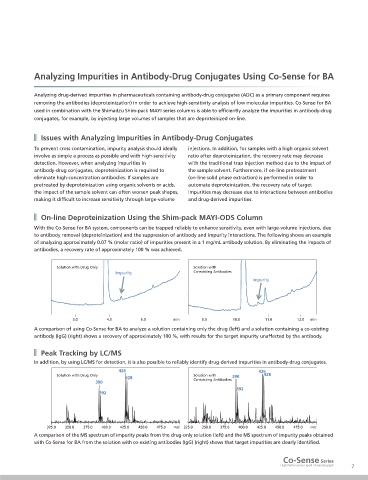
Solution with Drug Only Solution with
Impurity Co-existing Antibodies
Impurity
3.0 4.0 5.0 min 9.0 10.0 11.0 12.0 min
A comparison of using Co-Sense for BA to analyze a solution containing only the drug (left) and a solution containing a co-existing
antibody (IgG) (right) shows a recovery of approximately 100 %, with results for the target impurity unaffected by the antibody.
Peak Tracking by LC/MS
In addition, by using LC/MS for detection, it is also possible to reliably identify drug-derived impurities in antibody-drug conjugates.
426 426
Solution with Drug Only Solution with 390 428
428 Co-existing Antibodies
390
392
392
325.0 350.0 375.0 400.0 425.0 450.0 475.0 m/z 325.0 350.0 375.0 400.0 425.0 450.0 475.0 m/z
A comparison of the MS spectrum of impurity peaks from the drug-only solution (left) and the MS spectrum of impurity peaks obtained
with Co-Sense for BA from the solution with co-existing antibodies (IgG) (right) shows that target impurities are clearly identified.
Co-Sense Series
High Performance Liquid Chromatograph 7