Page 101 - Application Handbook - Liquid Chromatography
P. 101
Application No.L478
News
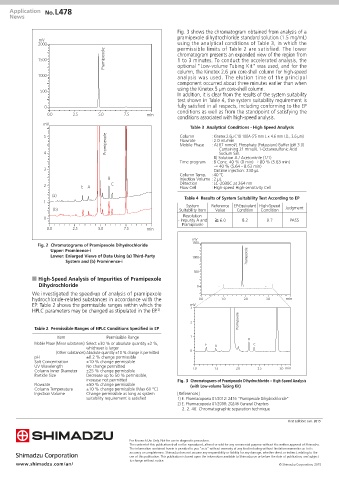
Fig. 3 shows the chromatogram obtained from analysis of a
pramipexole dihydrochloride standard solution (1.5 mg/mL)
mV
2000 using the analytical conditions of Table 3, in which the
permissible limits of Table 2 are satisfied. The lower
Pramipexole chromatogram presents an expanded view of the region from
1500 1 to 3 minutes. To conduct the accelerated analysis, the
optional "Low-volume Tubing Kit" was used, and for the
column, the Kinetex 2.6 µm core-shell column for high-speed
1000 analysis was used. The elution time of the principal
component occurred about three minutes earlier than when
using the Kinetex 5 µm core-shell column.
500
In addition, it is clear from the results of the system suitability
test shown in Table 4, the system suitability requirement is
0 fully satisfied in all respects, including conforming to the EP
conditions as well as from the standpoint of satisfying the
0.0 2.5 5.0 7.5 min
conditions associated with high-speed analysis.
mV
Table 3 Analytical Conditions - High Speed Analysis
Pramipexole Mobile Phase : A) 67 mmol/L Phosphate (Potassium) Buffer (pH 3.0)
5 Column : Kinetex 2.6µ C18 100A (75 mm L × 4.6 mm I.D., 2.6 µm)
: 2.0 mL/min
Flowrate
4 Containing 21 mmol/L 1-Octanesulfonic Acid
Sodium Salt
B) Solution A / Acetonitrile (1/1)
Time program : B Conc. 40 % (0 min) → 80 % (5.63 min)
→ 40 % (5.64 - 8.63 min)
3 Ontime injection: 230 µL
Column Temp. : 40 ˚C
B Injection Volume : 2 µL
2 E A C Detection : LC-2030C at 264 nm
Flow Cell
: High-speed High-sensitivity Cell
(a) Table 4 Results of System Suitability Test According to EP
1
System Reference EP-Equivalent High-Speed
(b) Suitability Item Value Condition Condition Judgment
Resolution
0
- Impurity A and ≧ 6.0 8.2 9.7 PASS
Pramipexole
0.0 2.5 5.0 7.5 min
mV
Fig. 2 Chromatograms of Pramipexole Dihydrochloride 1500
Upper: Prominence-i
Lower: Enlarged Views of Data Using (a) Third-Party 1000 Pramipexole
System and (b) Prominence-i
500
n High-Speed Analysis of Impurities of Pramipexole
Dihydrochloride 0
We investigated the speed-up of analysis of pramipexole
hydrochloride-related substances in accordance with the 0.0 1.0 2.0 3.0 min
EP. Table 2 shows the permissible ranges within which the mV
HPLC parameters may be changed as stipulated in the EP. 2) 3
Pramipexole
2
Table 2 Permissible Ranges of HPLC Conditions Specified in EP
Item Permissible Range 1 B
Mobile Phase (Minor substances) Select ±30 % or absolute quantity ±2 %, E A C
whichever is larger
(Other substances) Absolute quantity ±10 % change is permitted 0
pH ±0.2 % change permissible
Salt Concentration ±10 % change permissible
UV Wavelength No change permitted 1.0 1.5 2.0 2.5 3.0 min
Column Inner Diameter ±25 % change permissible
Particle Size Decrease up to 50 % permissible,
increase not permitted Fig. 3 Chromatograms of Pramipexole Dihydrochloride – High-Speed Analysis
Flowrate ±50 % change permissible (with Low-volume Tubing Kit)
Column Temperature ±10 % change permissible (Max 60 °C)
Injection Volume Change permissible as long as system [References]
suitability requirement is satisfied 1) E. Pharmacopoeia 01/2012: 2416 “Pramipexole Dihydrochloride”
2) E. Pharmacopoeia 01/2008: 20246 General Chapters
2. 2. 46. Chromatographic separation technique
First Edition: Jan. 2015
For Research Use Only. Not for use in diagnostic procedures.
The content of this publication shall not be reproduced, altered or sold for any commercial purpose without the written approval of Shimadzu.
The information contained herein is provided to you "as is" without warranty of any kind including without limitation warranties as to its
accuracy or completeness. Shimadzu does not assume any responsibility or liability for any damage, whether direct or indirect, relating to the
use of this publication. This publication is based upon the information available to Shimadzu on or before the date of publication, and subject
to change without notice.
www.shimadzu.com/an/ © Shimadzu Corporation, 2015