Page 99 - Application Handbook - Liquid Chromatography
P. 99
Application No.L466
News
Table 2 Results of USP System Suitability Test Using Duloxetine
(Fig. 1 (b) Prominence-i) mV
1500
System Suitability Requirements Criteria Observed Result
Resolution Between Duloxetine and 1000 Escitalopram
Duloxetine Related Compound F ≥ 1.5 4.2 PASS
Symmetry Factor for Duloxetine ≤ 1.5 1.3 PASS 500
Peak area %RSD for Duloxetine ≤ 1.0 0.17 PASS
0
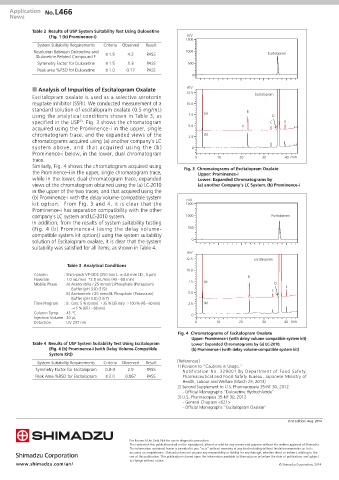
n Analysis of Impurities of Escitalopram Oxalate mV
12.5 Escitalopram
Escitalopram oxalate is used as a selective serotonin
reuptake inhibitor (SSRI). We conducted measurement of a 10.0
standard solution of escitalopram oxalate (0.5 mg/mL) B
using the analytical conditions shown in Table 3, as 7.5 (a) D
specified in the USP . Fig. 3 shows the chromatogram C E
3)
acquired using the Prominence-i in the upper, single 5.0 A
chromatogram trace, and the expanded views of the 2.5 (b)
chromatograms acquired using (a) another company's LC
system above, and that acquired using the (b) 0
Prominence-i below, in the lower, dual chromatogram
trace. 0 10 20 30 40 min
Similarly, Fig. 4 shows the chromatogram acquired using Fig. 3 Chromatograms of Escitalopram Oxalate
the Prominence-i in the upper, single chromatogram trace, Upper: Prominence-i
while in the lower, dual chromatogram trace, expanded Lower: Expanded Chromatograms by
views of the chromatogram obtained using the (a) LC-2010 (a) another Company's LC System, (b) Prominence-i
in the upper of the two traces, and that acquired using the
(b) Prominence-i with the delay volume-compatible system mV
kit option. From Fig. 3 and 4, it is clear that the 1500
Prominence-i has separation compatibility with the other
company's LC system and LC-2010 system. 1000 Escitalopram
In addition, from the results of system suitability testing
(Fig. 4 (b) Prominence-i (using the delay volume- 500
compatible system kit option)) using the system suitability
solution of Escitalopram oxalate, it is clear that the system 0
suitability was satisfied for all items, as shown in Table 4.
mV
12.5 Escitalopram
Table 3 Analytical Conditions
10.0
Column : Shim-pack VP-ODS (250 mm L. × 4.6 mm I.D., 5 µm) B
Flowrate : 1.0 mL/min *2.0 mL/min (45 - 60 min) 7.5 (a)
Mobile Phase : A) Acetonitrile / 25 mmol/L Phosphate (Potassium) D E
Buffer (pH 3.0) (1/9) C
B) Acetonitrile / 25 mmol/L Phosphate (Potassium) 5.0
Buffer (pH 3.0) (13/7)
Time Program : B. Conc. 5 % (0 min) → 35 % (35 min) → 100 % (45 - 60 min) 2.5 (b)
→ 5 % (60.1 - 68 min)
Column Temp. : 45 °C 0
Injection Volume : 20 µL
Detection : UV 237 nm 0 10 20 30 40 min
Fig. 4 Chromatograms of Escitalopram Oxalate
Upper: Prominence-i (with delay volume-compatible system kit)
Table 4 Results of USP System Suitability Test Using Escitalopram Lower: Expanded Chromatograms by (a) LC-2010,
(Fig. 4 (b) Prominence-i (with Delay Volume-Compatible (b) Prominence-i (with delay volume-compatible system kit)
System Kit))
System Suitability Requirements Criteria Observed Result [References]
1) Revision to "Cautions in Usage,"
Symmetry Factor for Escitalopram 0.8-3 2.9 PASS
Notification No. 329001 by Department of Food Safety,
Peak Area %RSD for Escitalopram ≤ 2.0 0.067 PASS Pharmaceutical and Food Safety Bureau, Japanese Ministry of
Health, Labour and Welfare (March 29, 2013)
2) Second Supplement to U.S. Pharmacopeia 35-NF 30, 2012
- Official Monographs "Duloxetine Hydrochloride"
3) U.S. Pharmacopeia 35-NF 30, 2012
- General Chapters <621>
- Official Monographs "Escitalopram Oxalate"
First Edition: Aug. 2014
For Research Use Only. Not for use in diagnostic procedures.
The content of this publication shall not be reproduced, altered or sold for any commercial purpose without the written approval of Shimadzu.
The information contained herein is provided to you "as is" without warranty of any kind including without limitation warranties as to its
accuracy or completeness. Shimadzu does not assume any responsibility or liability for any damage, whether direct or indirect, relating to the
use of this publication. This publication is based upon the information available to Shimadzu on or before the date of publication, and subject
to change without notice.
www.shimadzu.com/an/ © Shimadzu Corporation, 2014