Page 98 - Application Handbook - Liquid Chromatography
P. 98
LAAN-A-LC-E243
Application High Performance Liquid Chromatography
News Analysis of Impurities in New-generation Antidepressants
by Prominence-i
No.L466
Pharmaceutical companies in Japan are under notification
by the Ministry of Health, Labour and Welfare to carefully mV
consider the administration of the new-generation 750
antidepressant drugs that have been on the market since 500
1999 in Japan to patients under the age of 18 . Further, to Duloxetine
1)
ensure the properties and suitability of these drugs, they 250
are listed in the United States Pharmacopoeia (USP) and
European Pharmacopoeia (EP). 0
The new Prominence-i integrated high-performance liquid
chromatograph features separation compatibility with the
mV
systems of other companies. Further, use of the delay 60 C
volume-compatible system kit option provides separation Duloxetine
compatibility with the previous LC-2010 model, thereby 50 (a) D
permitting the smooth transfer of methods from the 40 B
currently used instrument.
Here, using the new Prominence-i integrated high- 30 E F
performance liquid chromatograph, we introduce an
example of analysis of compounds related to the 20 (b)
abovementioned new-generation antidepressants. 10
n Analysis of Impurities of Duloxetine Hydrochloride 0
0 10 20 30 min
Antidepressant drugs are psychotropic drugs that affect
neurotransmitters such as noradrenaline and serotonin that
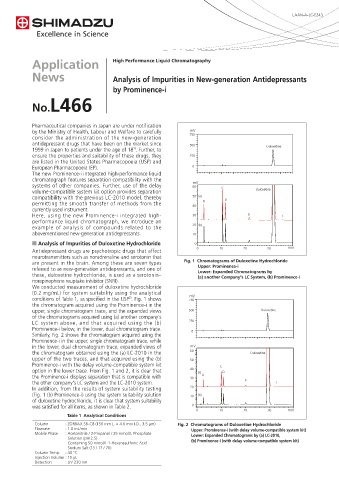
are present in the brain. Among these are seven types Fig. 1 Chromatograms of Duloxetine Hydrochloride
referred to as new-generation antidepressants, and one of Upper: Prominence-i
these, duloxetine hydrochloride, is used as a serotonin– Lower: Expanded Chromatograms by
(a) another Company's LC System, (b) Prominence-i
norepinephrine reuptake inhibitor (SNRI).
We conducted measurement of duloxetine hydrochloride
(0.2 mg/mL) for system suitability using the analytical
mV
2)
conditions of Table 1, as specified in the USP . Fig. 1 shows 750
the chromatogram acquired using the Prominence-i in the
upper, single chromatogram trace, and the expanded views 500 Duloxetine
of the chromatograms acquired using (a) another company's
LC system above, and that acquired using the (b) 250
Prominence-i below, in the lower, dual chromatogram trace. 0
Similarly, Fig. 2 shows the chromatogram acquired using the
Prominence-i in the upper, single chromatogram trace, while
in the lower, dual chromatogram trace, expanded views of mV
the chromatogram obtained using the (a) LC-2010 in the 60 Duloxetine
upper of the two traces, and that acquired using the (b) 50
Prominence-i with the delay volume-compatible system kit C
option in the lower trace. From Fig. 1 and 2, it is clear that 40 (a) D
the Prominence-i displays separation that is compatible with 30 B
the other company's LC system and the LC-2010 system. E F
In addition, from the results of system suitability testing 20
(Fig. 1 (b) Prominence-i) using the system suitability solution 10 (b)
of duloxetine hydrochloride, it is clear that system suitability
was satisfied for all items, as shown in Table 2. 0
0 10 20 30 min
Table 1 Analytical Conditions
Column : ZORBAX SB-C8 (150 mm L. × 4.6 mm I.D., 3.5 µm) Fig. 2 Chromatograms of Duloxetine Hydrochloride
Flowrate : 1.0 mL/min Upper: Prominence-i (with delay volume-compatible system kit)
Mobile Phase : Acetonitrile / 2-Propanol / 25 mmol/L Phosphate Lower: Expanded Chromatograms by (a) LC-2010,
Solution (pH 2.5) (b) Prominence-i (with delay volume-compatible system kit)
Containing 50 mmol/L 1-Hexanesulfonic Acid
Sodium Salt (13 / 17 / 70)
Column Temp. : 40 °C
Injection Volume : 10 µL
Detection : UV 230 nm