Page 105 - Application Handbook - Liquid Chromatography
P. 105
Application No.L457
News
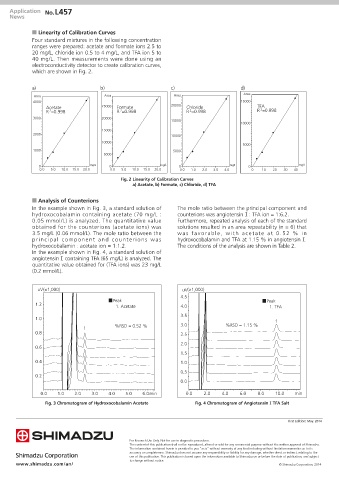
n Linearity of Calibration Curves
Four standard mixtures in the following concentration
ranges were prepared: acetate and formate ions 2.5 to
20 mg/L, chloride ion 0.5 to 4 mg/L, and TFA ion 5 to
40 mg/L. Then measurements were done using an
electroconductivity detector to create calibration curves,
which are shown in Fig. 2.
a) b) c) d)
Area Area Area Area
4000 15000
Acetate 25000 Formate 20000 Chloride TFA
2
2
2
R =0.998 R =0.998 R =0.998 R =0.998
2
3000 20000
15000
10000
15000
2000 10000
10000
5000
1000 5000
5000
0 mg/L 0 mg/L 0 mg/L 0 mg/L
0.0 5.0 10.0 15.0 20.0 0.0 5.0 10.0 15.0 20.0 0.0 1.0 2.0 3.0 4.0 0 10 20 30 40
Fig. 2 Linearity of Calibration Curves
a) Acetate, b) Formate, c) Chloride, d) TFA
n Analysis of Counterions
In the example shown in Fig. 3, a standard solution of The mole ratio between the principal component and
hydroxocobalamin containing acetate (70 mg/L : counterions was angiotensin Ⅰ : TFA ion = 1:6.2.
0.05 mmol/L) is analyzed. The quantitative value Furthermore, repeated analysis of each of the standard
obtained for the counterions (acetate ions) was solutions resulted in an area repeatability (n = 6) that
3.5 mg/L (0.06 mmol/L). The mole ratio between the was favorable, with acetate at 0.52 % i n
principal component and counterions was hydroxocobalamin and TFA at 1.15 % in angiotensin Ⅰ.
hydroxocobalamin : acetate ion = 1:1.2. The conditions of the analysis are shown in Table 2.
In the example shown in Fig. 4, a standard solution of
angiotensin Ⅰ containing TFA (65 mg/L) is analyzed. The
quantitative value obtained for (TFA ions) was 23 mg/L
(0.2 mmol/L).
uV(×1,000) uV(×1,000)
4.5
˙Peak ˙Peak
1.2
1. Acetate 4.0 1. TFA
3.5
1.0 1
%RSD = 0.52 % 3.0 %RSD = 1.15 %
1
0.8 2.5
2.0
0.6
1.5
0.4 1.0
0.5
0.2
0.0
0.0 1.0 2.0 3.0 4.0 5.0 6.0min 0.0 2.0 4.0 6.0 8.0 10.0 min
Fig. 3 Chromatogram of Hydroxocobalamin Acetate Fig. 4 Chromatogram of Angiotensin Ⅰ TFA Salt
First Edition: May. 2014
For Research Use Only. Not for use in diagnostic procedures.
The content of this publication shall not be reproduced, altered or sold for any commercial purpose without the written approval of Shimadzu.
The information contained herein is provided to you "as is" without warranty of any kind including without limitation warranties as to its
accuracy or completeness. Shimadzu does not assume any responsibility or liability for any damage, whether direct or indirect, relating to the
use of this publication. This publication is based upon the information available to Shimadzu on or before the date of publication, and subject
to change without notice.
www.shimadzu.com/an/ © Shimadzu Corporation, 2014