Page 107 - Application Handbook - Liquid Chromatography
P. 107
Application No.L504
News
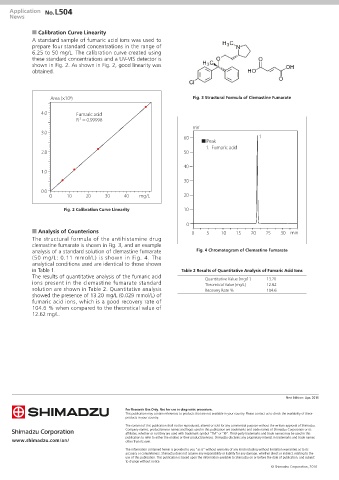
n Calibration Curve Linearity
A standard sample of fumaric acid ions was used to
prepare four standard concentrations in the range of
6.25 to 50 mg/L. The calibration curve created using
these standard concentrations and a UV-VIS detector is
shown in Fig. 2. As shown in Fig. 2, good linearity was
obtained.
Area (×10 ) Fig. 3 Structural Formula of Clemastine Fumarate
6
4.0 Fumaric acid
2
R = 0.99998
mV
3.0
60 1
■Peak
1. Fumaric acid
2.0 50
40
1.0
30
0.0
0 10 20 30 40 mg/L 20
Fig. 2 Calibration Curve Linearity 10
0
n Analysis of Counterions 0 5 10 15 20 25 30 min
The structural formula of the antihistamine drug
clemastine fumarate is shown in Fig. 3, and an example
analysis of a standard solution of clemastine fumarate Fig. 4 Chromatogram of Clemastine Fumarate
(50 mg/L: 0.11 mmol/L) is shown in Fig. 4. The
analytical conditions used are identical to those shown
in Table 1. Table 2 Results of Quantitative Analysis of Fumaric Acid Ions
The results of quantitative analysis of the fumaric acid Quantitative Value [mg/L] 13.20
ions present in the clemastine fumarate standard Theoretical Value [mg/L] 12.62
solution are shown in Table 2. Quantitative analysis Recovery Rate % 104.6
showed the presence of 13.20 mg/L (0.029 mmol/L) of
fumaric acid ions, which is a good recovery rate of
104.6 % when compared to the theoretical value of
12.62 mg/L.
First Edition: Apr. 2016
For Research Use Only. Not for use in diagnostic procedure.
This publication may contain references to products that are not available in your country. Please contact us to check the availability of these
products in your country.
The content of this publication shall not be reproduced, altered or sold for any commercial purpose without the written approval of Shimadzu.
Company names, product/service names and logos used in this publication are trademarks and trade names of Shimadzu Corporation or its
affiliates, whether or not they are used with trademark symbol “TM” or “®”. Third-party trademarks and trade names may be used in this
publication to refer to either the entities or their products/services. Shimadzu disclaims any proprietary interest in trademarks and trade names
www.shimadzu.com/an/ other than its own.
The information contained herein is provided to you "as is" without warranty of any kind including without limitation warranties as to its
accuracy or completeness. Shimadzu does not assume any responsibility or liability for any damage, whether direct or indirect, relating to the
use of this publication. This publication is based upon the information available to Shimadzu on or before the date of publication, and subject
to change without notice.
© Shimadzu Corporation, 2016