Page 14 - Pharmaceutical M5 Biopharma
P. 14
14 Sponsored Feature
Bioanalysis Accelerating the pre-clinical/clinical phase
Bioanalytical method development is the critical step in the biopharmaceutical pipeline as it bridges the transition to pre-clinical
and clinical phases. Ligand-binding assay (LBA) has been the common technique for biologics, however, LC-MS is emerging as an
alternative to reduce time and cost needed for method development and to gain increased selectivity and efficiency.
To further simplify and streamline the LCMS workflow for antibody bioanalysis, Shimadzu developed an innovative nanotechnology-
based nSMOL™ Antibody Bioanalysis platform for the selective proteolysis of the Fab region of antibody drugs. This increases the
detection sensitivity of surrogate peptides in CDR regions, which can be accurately quantified via MRM measurements using a triple
quadrupole high performance liquid chromatograph mass spectrometer.
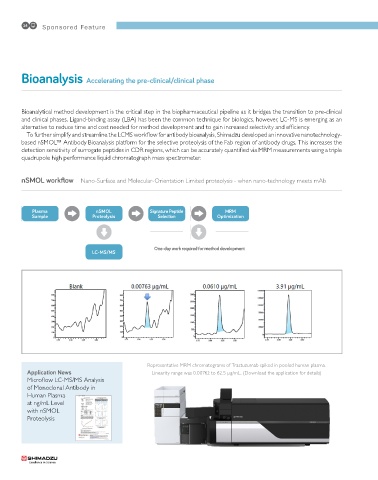
nSMOL workflow Nano-Surface and Molecular-Orientation Limited proteolysis - when nano-technology meets mAb
Plasma nSMOL Signature Peptide MRM
Sample Proteolysis Selection Optimization
One-day work required for method development
LC-MS/MS
Representative MRM chromatograms of Trastuzumab spiked in pooled human plasma.
Application News Linearity range was 0.00762 to 62.5 μg/mL. (Download the application for details)
Microflow LC-MS/MS Analysis
of Monoclonal Antibody in
Human Plasma
at ng/mL Level
with nSMOL
Proteolysis