Page 55 - Pharmaceutical Solution for Pharma Analysis
P. 55
Application No.M272
News
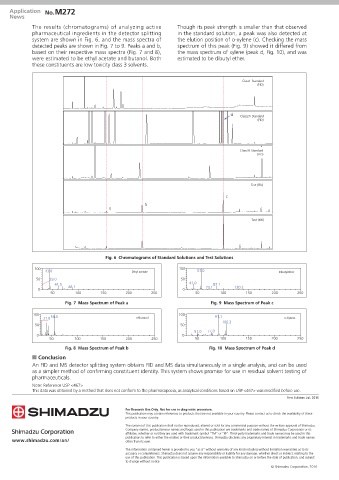
The results (chromatograms) of analyzing active Though its peak strength is smaller than that observed
pharmaceutical ingredients in the detector splitting in the standard solution, a peak was also detected at
system are shown in Fig. 6, and the mass spectra of the elution position of o-xylene (c). Checking the mass
detected peaks are shown in Fig. 7 to 9. Peaks a and b, spectrum of this peak (Fig. 9) showed it differed from
based on their respective mass spectra (Fig. 7 and 8), the mass spectrum of xylene (peak d, Fig. 10), and was
were estimated to be ethyl acetate and butanol. Both estimated to be dibutyl ether.
these constituents are low toxicity class 3 solvents.
Class1 Standard
(FID)
5.0 10.0 15.0 20.0 25.0 d 30.0 35.0
Class2A Standard
(FID)
5.0 10.0 15.0 20.0 25.0 30.0 35.0
Class2B Standard
(FID)
5.0 10.0 15.0 20.0 25.0 30.0 Test (FID) 35.0
c
b
a
5.0 10.0 15.0 20.0 25.0 30.0 Test (MS) 35.0
Fig. 6 Chromatograms of Standard Solutions and Test Solutions
100 43.0 Ethyl acetate 100 57.0 Dibutylether
50 29.0 50
61.0 41.0 87.1
0 88.1 0 73.1 130.2
50 100 150 200 250 50 100 150 200 250
Fig. 7 Mass Spectrum of Peak a Fig. 9 Mass Spectrum of Peak c
100 56.0 100 91.1
31.0 n-Butanol o-Xylene
106.2
50 50
51.0 77.0
0 0
50 100 150 200 250 50 100 150 200 250
Fig. 8 Mass Spectrum of Peak b Fig. 10 Mass Spectrum of Peak d
n Conclusion
An FID and MS detector splitting system obtains FID and MS data simultaneously in a single analysis, and can be used
as a simpler method of confirming constituent identity. This system shows promise for use in residual solvent testing of
pharmaceuticals.
Note: Reference USP <467>
This data was obtained by a method that does not conform to the pharmacopoeia, as analytical conditions based on USP <467> was modified before use.
First Edition: Jul. 2016
For Research Use Only. Not for use in diagnostic procedure.
This publication may contain references to products that are not available in your country. Please contact us to check the availability of these
products in your country.
The content of this publication shall not be reproduced, altered or sold for any commercial purpose without the written approval of Shimadzu.
Company names, product/service names and logos used in this publication are trademarks and trade names of Shimadzu Corporation or its
affiliates, whether or not they are used with trademark symbol “TM” or “®”. Third-party trademarks and trade names may be used in this
publication to refer to either the entities or their products/services. Shimadzu disclaims any proprietary interest in trademarks and trade names
www.shimadzu.com/an/ other than its own.
The information contained herein is provided to you "as is" without warranty of any kind including without limitation warranties as to its
accuracy or completeness. Shimadzu does not assume any responsibility or liability for any damage, whether direct or indirect, relating to the
use of this publication. This publication is based upon the information available to Shimadzu on or before the date of publication, and subject
to change without notice.
© Shimadzu Corporation, 2016