Page 60 - Pharmaceutical Solution for Pharma Analysis
P. 60
C146-E339
High-Sensitivity Analysis of Genotoxic Impurities: GCMS-TQ8050
Genotoxic (mutagenic) impurities must be controlled to low
concentration levels. Therefore, high-sensitivity analytical instruments
are required for their analysis. Triple quadrupole gas chromatograph
mass spectrometers (GC-MS/MS) offer significantly higher selectivity by
separating components by mass in two stages. Consequently, they are
able to selectively detect trace quantities of genotoxic impurities with GCMS Solutions for Pharmaceuticals
high sensitivity.
To maximize the benefits of OFF-AXIS ion optics, including both high
ion transmission performance and outstanding noise elimination
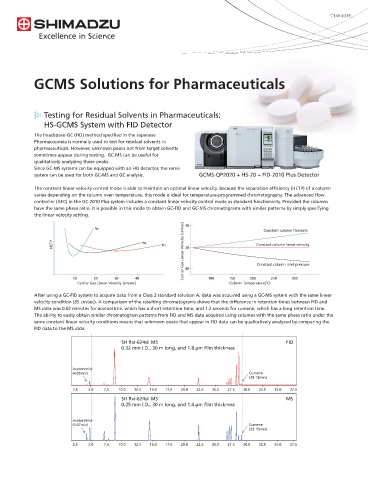
performance, the GCMS-TQ8050 now features a detector with higher Testing for Residual Solvents in Pharmaceuticals:
amplification performance that achieves the world's highest HS-GCMS System with FID Detector
sensitivity*.
* As of August 2016, according to a Shimadzu survey The headspace-GC (FID) method specified in the Japanese
Triple Quadrupole
Gas Chromatograph Mass Spectrometer Pharmacopoeia is normally used to test for residual solvents in
The GCMS-TQ8050 supports measurements as a single-MS system (scan pharmaceuticals. However, unknown peaks not from target solvents
and SIM modes). It is also compatible with almost everything GCMS-TQ8050 sometimes appear during testing. GC-MS can be useful for
supported by the GCMS-QP2020, including those mentioned above qualitatively analyzing those peaks.
(FID detector, software, DI unit, and Smart EI/CI ion source). Thus, users Since GC-MS systems can be equipped with an FID detector, the same
can configure systems according to their purposes. system can be used for both GC-MS and GC analysis. GCMS-QP2020 + HS-20 + FID-2010 Plus Detector
The constant linear velocity control mode is able to maintain an optimal linear velocity. Because the separation efficiency (HETP) of a column
varies depending on the column oven temperature, this mode is ideal for temperature-programmed chromatography. The advanced flow
GC-MS (SIM) GC-MS/MS (MRM) controller (AFC) in the GC-2010 Plus system includes a constant linear velocity control mode as standard functionality. Provided the columns
S/N:31 S/N:1960 have the same phase ratio, it is possible in this mode to obtain GC-FID and GC-MS chromatograms with similar patterns by simply specifying
7.5
the linear velocity setting.
2.0
60 times 30
higher sensitivity N2 Constant column flowrate
5.0
HETP H2 Carrier Gas Linear Velocity (cm/sec) 20 Constant column linear velocity
1.5 He
2.5
1.0 40 Constant column inlet pressure
10 20 30 40 100 150 200 250 300
Carrier Gas Linear Velocity (cm/sec) Column Temperature(˚C)
Ethyl methanesulfonate
GC-MS (SIM) GC-MS/MS (MRM) After using a GC-FID system to acquire data from a Class 2 standard solution A, data was acquired using a GC-MS system with the same linear
velocity condition (35 cm/sec). A comparison of the resulting chromatograms shows that the difference in retention times between FID and
S/N:45 5.0 S/N:2467
3.0 MS data was 0.02 minutes for acetonitrile, which has a short retention time, and 1.2 seconds for cumene, which has a long retention time.
The ability to easily obtain similar chromatogram patterns from FID and MS data acquired using columns with the same phase ratio under the
50 times 4.0 same constant linear velocity conditions means that unknown peaks that appear in FID data can be qualitatively analyzed by comparing the
higher sensitivity FID data to the MS data.
2.0 3.0
SH Rxi-624sil MS FID
2.0 0.32 mm I.D., 30 m long, and 1.8 µm film thickness
1.0
1.0
Acetonitrile
(4.05min) Cumene
(29.13min)
n-Propyl p-toluene sulfonate
2.5 5.0 7.5 10.0 12.5 15.0 17.5 20.0 22.5 25.0 27.5 30.0 32.5 35.0 37.5
Solution concentration: 0.01 µg/mL [equivalent to a 1 ng/mg
(1 ppm) concentration in pharmaceutical drug substances] SH Rxi-624sil MS MS
0.25 mm I.D., 30 m long, and 1.4 µm film thickness
For Research Use Only. Not for use in diagnostic procedures. Acetonitrile
This publication may contain references to products that are not available in your country. Please contact us to check the availability of these (4.07min) Cumene
products in your country. (29.15min)
Company names, products/service names and logos used in this publication are trademarks and trade names of Shimadzu Corporation, its
subsidiaries or its affiliates, whether or not they are used with trademark symbol “TM” or “®”.
Third-party trademarks and trade names may be used in this publication to refer to either the entities or their products/services, whether or not
they are used with trademark symbol “TM” or “®”. 2.5 5.0 7.5 10.0 12.5 15.0 17.5 20.0 22.5 25.0 27.5 30.0 32.5 35.0 37.5
Shimadzu disclaims any proprietary interest in trademarks and trade names other than its own.
www.shimadzu.com/an/ The contents of this publication are provided to you “as is” without warranty of any kind, and are subject to change without notice. Shimadzu
does not assume any responsibility or liability for any damage, whether direct or indirect, relating to the use of this publication.
© Shimadzu Corporation, 2017
Printed in Japan 3655-02723-20AIT