Page 29 - Clinical-Medical Solution for Clinical Chemistry
P. 29
Application AD-0135
News
Area Ratio Area Ratio Table 6: Recovery (%) of five β-lactam antibiotics in plasma samples
30 (1) MER / MER-d6 Zoomed by protein crash pre-treatment and determined on LCMS-8060
3
25
Recovery (%)
20 2 Compd. 20 40 80 200 400 2000 4000
15 ng/mL ng/mL ng/mL ng/mL ng/mL ng/mL ng/mL
1
10 TAZ 86 89 90 94 97 92 97
0 CEF 94 87 87 88 90 88 89
5
0.0 1.0 Conc. Ratio
MER 103 104 98 102 102 97 103
0
0.0 5.0 10.0 Conc. Ratio CFT 113 94 93 97 103 100 101
Area Ratio Area Ratio
PIP 104 106 98 105 103 98 102
7 (2) TAZ / MER-d6 (3) CEF / CEF-cd3
6 15 Matrix effect of the method was determined by the peak area
ratios of spiked samples and mixed standards in pure solvent
5
at all concentration levels. The results are shown in Table 7.
4 10
It can be seen that strong matrix effect occurred for CFT
3
(33%~40%) and TAZ (128%~149%). This could be due to
2 5
interference from plasma, which causes ion suppression and
1
ion amplification. By further dilution of 2.5 times of the plasma
0 0
0.0 5.0 10.0 15.0 Conc. Ratio 0.0 2.5 Conc. Ratio samples with pure water before injection into LCMSMS, the
Area Ratio Area Ratio matrix effects of CFT and TAZ were improved significantly to
15.0
7.5 (4) CFT / CFT-d6 (5) PIP / PIP-d5 62%~85% and 95%~109%, respectively.
12.5
Table 7: Results of matrix effect (%) of five β-lactam antibiotics in
10.0
5.0
plasma samples on LCMS-8060
7.5
Matrix effect (%)
2.5 5.0
Compd. 20 40 80 200 400 2000 4000
2.5
ng/mL ng/mL ng/mL ng/mL ng/mL ng/mL ng/mL
0.0 0.0
0.0 2.5 Conc. Ratio 0.0 5.0 10.0 Conc. Ratio TAZ* 149 140 145 139 137 134 128
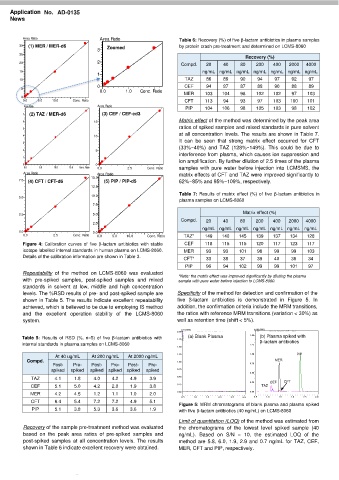
Figure 4: Calibration curves of five β-lactam antibiotics with stable CEF 118 115 115 120 117 123 117
isotope labelled internal standards in human plasma on LCMS-8060. MER 93 93 101 98 99 99 103
Details of the calibration information are shown in Table 3. CFT* 33 38 37 39 40 36 34
PIP 96 94 102 99 99 101 97
Repeatability of the method on LCMS-8060 was evaluated *Note: the matrix effect was improved significantly by diluting the plasma
with pre-spiked samples, post-spiked samples and mixed sample with pure water before injection to LCMS-8060.
standards in solvent at low, middle and high concentration
levels. The %RSD results of pre- and post-spiked sample are Specificity of the method for detection and confirmation of the
shown in Table 5. The results indicate excellent repeatability five β-lactam antibiotics is demonstrated in Figure 5. In
achieved, which is believed to be due to employing IS method addition, the confirmation criteria include the MRM transitions,
and the excellent operation stability of the LCMS-8060 the ratios with reference MRM transitions (variation < 30%) as
system. well as retention time (shift < 5%).
(x10,000) (x100,000)
2.00 1:Meropenem 384.10>68.10(+) CE: -41.0 1:Meropenem 384.10>68.10(+) CE: -41.0
2:Tazobactam 301.10>168.15(+) CE: -15.0 1.50 3:Cef epime 481.10>86.15(+) CE: -15.0
2:Tazobactam 301.10>168.15(+) CE: -15.0
(a) Blank Plasma
(b) Plasma spiked with
3:Cef epime 481.10>86.15(+) CE: -15.0
Table 5: Results of RSD (%, n=5) of five β-lactam antibiotics with 1.75 5:Piperacillin 518.20>143.10(+) CE: -21.0 4:Cef tazidime 547.10>468.00(+) CE: -13.0
4:Cef tazidime 547.10>468.00(+) CE: -13.0
5:Piperacillin 518.20>143.10(+) CE: -21.0
internal standards in plasma samples on LCMS-8060 1.25 β-lactam antibiotics
1.50
At 40 ug/mL At 200 ng/mL At 2000 ng/mL 1.25 1.00 PIP
Compd. 1.00 MER
Post- Pre- Post- Pre- Post- Pre- 0.75
spiked spiked spiked spiked spiked spiked 0.75
0.50
TAZ 4.1 1.8 4.0 4.2 4.9 3.9 0.50
0.25 CEF CFT
CEF 5.1 5.0 4.2 2.0 1.9 3.8 0.25 TAZ
MER 4.2 4.5 1.2 1.1 1.0 2.0 0.00 0.00
0.5 1.0 1.5 2.0 2.5 min 0.5 1.0 1.5 2.0 2.5 min
CFT 6.4 5.4 7.2 7.2 4.9 5.1
Figure 5: MRM chromatograms of blank plasma and plasma spiked
PIP 5.1 3.8 5.3 3.6 3.6 1.9 with five β-lactam antibiotics (40 ng/mL) on LCMS-8060
Limit of quantitation (LOQ) of the method was estimated from
Recovery of the sample pre-treatment method was evaluated the chromatograms of the lowest level spiked sample (40
based on the peak area ratios of pre-spiked samples and ng/mL). Based on S/N = 10, the estimated LOQ of the
post-spiked samples at all concentration levels. The results method are 5.8, 6.0, 1.9, 2.9 and 0.7 ng/mL for TAZ, CEF,
shown in Table 6 indicate excellent recovery were obtained. MER, CFT and PIP, respectively.