Page 36 - Shimadzu Journal vol.9 Issue2
P. 36
Since all the culturing steps are easy and safe, HYDROX offers an excellent alternative
material to animal-based ingredients, so Shimadzu intends to commercialize it as a prod- HYDROX
2 mg/mL
uct from 2023. As part of the commercialization process, Shimadzu Corporation partnered
with Osaka University in joint research using HYDROX nanofibers to efficiently obtain
hepatocyte-like cells from human iPS cells by applying hepatic differentiation or to main-
tain hepatic-specific functions of primary-cultured hepatocyte cells. That research demon-
strated that HYDROX can be used to form cell aggregates with enhanced functional
characteristics and can be used to improve the various liver functions of differentiated
cells (reference 2) .
In addition to using the HYDROX 3D nanofiber to enable easy three-dimensional cul-
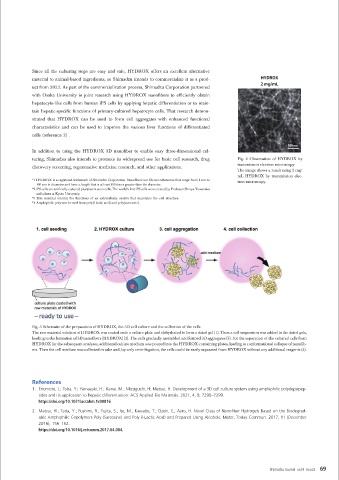
turing, Shimadzu also intends to promote its widespread use for basic cell research, drug Fig. 2 Observation of HYDROX by
transmission electron microscopy
discovery screening, regenerative medicine research, and other applications.
The image shows a result using 2 mg/
mL HYDROX by transmission elec-
*1 HYDROX is a registered trademark of Shimadzu Corporation. Nanofibers are fibrous substances that range from 1 nm to tron microscopy.
100 nm in diameter and have a length that is at least 100 times greater than the diameter.
*2 iPS cells are artificially cultured pluripotent stem cells. The world’s first iPS cells were created by Professor Shinya Yamanaka
and others at Kyoto University.
*3 This material mimics the functions of an extracellular matrix that maintains the cell structure.
*4 Amphiphilic polymer formed from poly(l-lactic acid) and poly(sarcosine).
Fig. 3 Schematic of the preparation of HYDROX, the 3D cell culture and the collection of the cells
The raw material solution of HYDROX was coated onto a culture plate and dehydrated to form a dried gel (1). Then a cell suspension was added to the dried gels,
leading to the formation of 3D nanofibers (HYDROX) (2). The cells gradually assembled and formed 3D aggregates (3). For the separation of the cultured cells from
HYDROX for the subsequent analyses, additional culture medium was poured into the HYDROX-containing plates, leading to conformational collapse of nanofib-
ers. Then the cell medium was collected in tube and, by only centrifugation, the cells could be easily separated from HYDROX without any additional reagents (4).
References
1. Enomoto, J.; Toba, Y.; Yamazaki, H.; Kanai, M.; Mizuguchi, H; Matsui, H. Development of a 3D cell culture system using amphiphilic polydepsipep
tides and its application to hepatic differentiation. ACS Applied Bio Materials. 2021, 4, 9, 7290–7299.
https://doi.org/10.1021/acsabm.1c00816
2. Matsui, H.; Tada, Y.; Fushimi, R.; Fujita, S.; Ito, M.; Kawabe, T.; Ozeki, E.; Ajiro, H. Novel Class of Nanofiber Hydrogels Based on the Bio degrad
able Amphiphilic Copolymers Poly (Sarcosine) and Poly (lLactic Acid) and Prepared Using Alcohols. Mater. Today Commun. 2017, 11 (December
2016), 156–162.
https://doi.org/10.1016/j.mtcomm.2017.04.004.
Shimadzu Journal vol.9 Issue2 69