Page 11 - Shimadzu ICPMS-2030 Series
P. 11
Pharmaceuticals/Pharmacopoeia
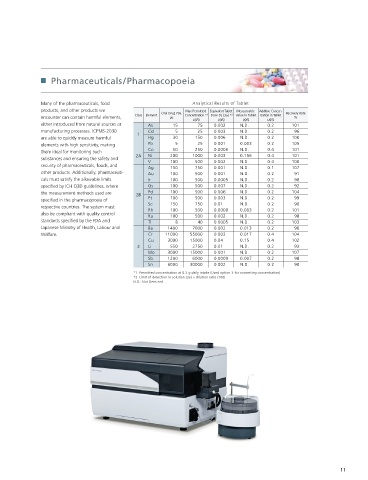
Many of the pharmaceuticals, food Analytical Results of Tablet
products, and other products we Oral Drug PDE Max Permitted Equivalent Tablet Measureable Additive Concen- Recovery Rate
encounter can contain harmful elements, Class Element µg Concentration * 1 Dose DL (3σ) * 2 Value in Tablet tration in Tablet %
µg/g µg/g µg/g µg/g
either introduced from natural sources or As 15 75 0.002 N.D. 0.2 101
manufacturing processes. ICPMS-2030 Cd 5 25 0.003 N.D. 0.2 96
1
are able to quickly measure harmful Hg 30 150 0.006 N.D. 0.2 100
elements with high sensitivity, making Pb 5 25 0.001 0.003 0.2 105
them ideal for monitoring such Co 50 250 0.0006 N.D. 0.4 101
2A Ni 200 1000 0.003 0.156 0.4 101
substances and ensuring the safety and V 100 500 0.002 N.D. 0.4 100
security of pharmaceuticals, foods, and
Ag 150 750 0.001 N.D. 0.1 107
other products. Additionally, pharmaceuti- Au 100 500 0.001 N.D. 0.2 91
cals must satisfy the allowable limits Ir 100 500 0.0005 N.D. 0.2 98
speci ed by ICH Q3D guidelines, where Os 100 500 0.007 N.D. 0.2 92
the measurement methods used are 2B Pd 100 500 0.006 N.D. 0.2 104
speci ed in the pharmacopoeia of Pt 100 500 0.003 N.D. 0.2 99
Se 150 750 0.01 N.D. 0.2 98
respective countries. The system must Rh 100 500 0.0008 0.003 0.2 101
also be compliant with quality control Ru 100 500 0.002 N.D. 0.2 98
standards speci ed by the FDA and Tl 8 40 0.0005 N.D. 0.2 103
Japanese Ministry of Health, Labour and Ba 1400 7000 0.002 0.013 0.2 96
Welfare. Cr 11000 55000 0.003 0.017 0.4 104
Cu 3000 15000 0.04 0.15 0.4 102
3 Li 550 2750 0.01 N.D. 0.2 93
Mo 3000 15000 0.001 N.D. 0.2 107
Sb 1200 6000 0.0009 0.007 0.2 98
Sn 6000 30000 0.002 N.D. 0.2 98
*1 Permitted concentration at 0.2 g daily intake (Used option 3 for converting concentration)
*2 Limit of detection in solution (3σ) × dilution ratio (100)
N.D.: Not Detected
11